Scale-up of a perfusion-based dendritic cell generation process
Cell Gene Therapy Insights 2018; 4(11), 1117-1130.
10.18609/cgti.2018.110
Scale up of dendritic cell production is a critical challenge that is infeasible with current static culture systems such as well plates, T-flasks, and bags. We have developed a fully enclosed, sterile cell culture system, called EDEN, that allows for continuous perfusion of fresh differentiation medium into the cell culture cartridge and simultaneous removal of depleted medium. EDEN generated ca. 25 million immature dendritic cells (iDCs) per run with a yield, relative to seeded monocytes, of 20-30%. Immunophenotyping showed that EDEN generated iDCs were phenotypically similar to 6-well plate generated iDCs. Maturation of EDEN iDCs using a standard maturation cocktail was successful with upregulation of CD80/83/86 and downregulation of CD209. Computational fluid dynamics simulations aided the EDEN cartridge design to ensure proper differentiation medium perfusion. These results indicate that EDEN successfully generates clinically relevant numbers of iDCs in a single cell culture cartridge with fewer manual interventions compared to standard culture techniques.
Introduction
Generating clinically relevant numbers of monocyte-derived dendritic cells (MO-DCs) for therapeutic use can be challenging for both for research and clinical scale production. Standard well plate and T-flask culture is a cumbersome process with many manual steps that expose the cell culture to the outside environment. Each manual step requires exposing the cell culture to the outside (aseptic) environment and requires intervention by a highly trained technician, consuming valuable time and resources in the rapidly expanding field of cell therapy production. Although the manual steps are performed aseptically in a laminar flow hood, there are numerous safety and contamination concerns such as patient sample mix-up and misidentification, exposure to unknown contaminants inside the laminar flow hood (e.g., particulates and bacteria/fungus resistant to standard sterilization techniques such as 70% ethanol), and accidental exposure of culture to a septic environment [1]. Furthermore, numerous well plates are required to generate sufficient DCs for a single therapeutic dose. Alternative DC generation vessels include T-flasks and bags which reduce the number of culture vessels compared to well plates but also have the same inherent issues above as well as low DC yield in bags. Immature DC yield in static culture vessels ranges from ca. 4-41% in gas-permeable bags when MOs are positively selected. This range is also expected for well plates and T flasks and is dependent on culture conditions and donor [2–7]. Scale-up of manual DC generation techniques is generally not feasible aside from adding more culture vessels to the workflow.
Dosing regimens for DC vaccines vary widely between the type of study being conducted and the targeted disease; however, most DC vaccine regimens require >100 million autologous DCs per patient. Each therapeutic dose is administered at least 3 times, thus requiring 30–50+ well plates or numerous T flasks for a single patient. It is difficult to ascertain the exact number of well plates or flasks required for generating DCs from a single patient because this is dependent upon precursor cell (peripheral blood mononuclear cell [PBMC] or MO) seeding density, cytokine concentration, and final yield of generated DCs which are often times not specified. It is also well known that generating DCs from PBMCs or MOs of cancer patients often times leads to lower DC yields than generating DCs from healthy donors.
Carreno et al. investigated a melanoma DC vaccine regimen of 135 million DCs in the priming dose followed by two additional doses of 45 million DCs. The DCs were cultured from peripheral blood MOs for 6 days in tissue culture flasks followed by maturation for 24 hours in new flasks [8–11]. Mitchell et al. investigated a glioblastoma DC vaccine regimen of four bi-weekly doses followed by at least six subsequent monthly doses of 20 million DCs per dose. The DCs were cultured from peripheral blood MOs for: (a) 5 days in tissue culture flasks followed by 3–4 days of maturation in the same flasks or (b) 7 days in tissue culture flasks followed by 16–20 hours of maturation in new flasks [12,13]. Additionally, these protocols typically involve supplementing the cell culture with fresh differentiation medium multiple times during DC generation. It’s important to note that these are two examples of clinical DC dose regimens and other regimens have been evaluated [14,15].
To address sterility, contamination, and workflow issues associated with DC generation, we developed the MicroDEN system for smaller scale DC generation. This automated cell culture system continuously perfuses fresh medium into a culture vessel while simultaneously removing depleted medium [16]. The aseptic design of MicroDEN allows for fresh complete medium (base medium + cytokines) to be added into an inlet bottle that feeds to a peristaltic pump, through the culture vessel, and out into a waste bottle. Aseptic medium addition to the inlet bottle is achieved using Luer activated valves (LAVs) and stopcocks that are simply wiped clean with a standard alcohol wipe; this technique is heavily utilized in intravenous (IV) lines and anesthesia administration. Medium refresh is achieved using the same aseptic procedure. Aseptic cell seeding and harvesting is also incorporated to ensure sterility and minimize contamination sources of the final DC product. MicroDEN was designed to generate DCs on the scale of well plates and T-flasks, but scaleup is generally not feasible.
Using MicroDEN technology as a basis for workflow, we have developed an automated cell culture system for aseptically generating therapeutically relevant numbers of immature DCs (iDCs) in a single cell culture cartridge, called EDEN (Figure 1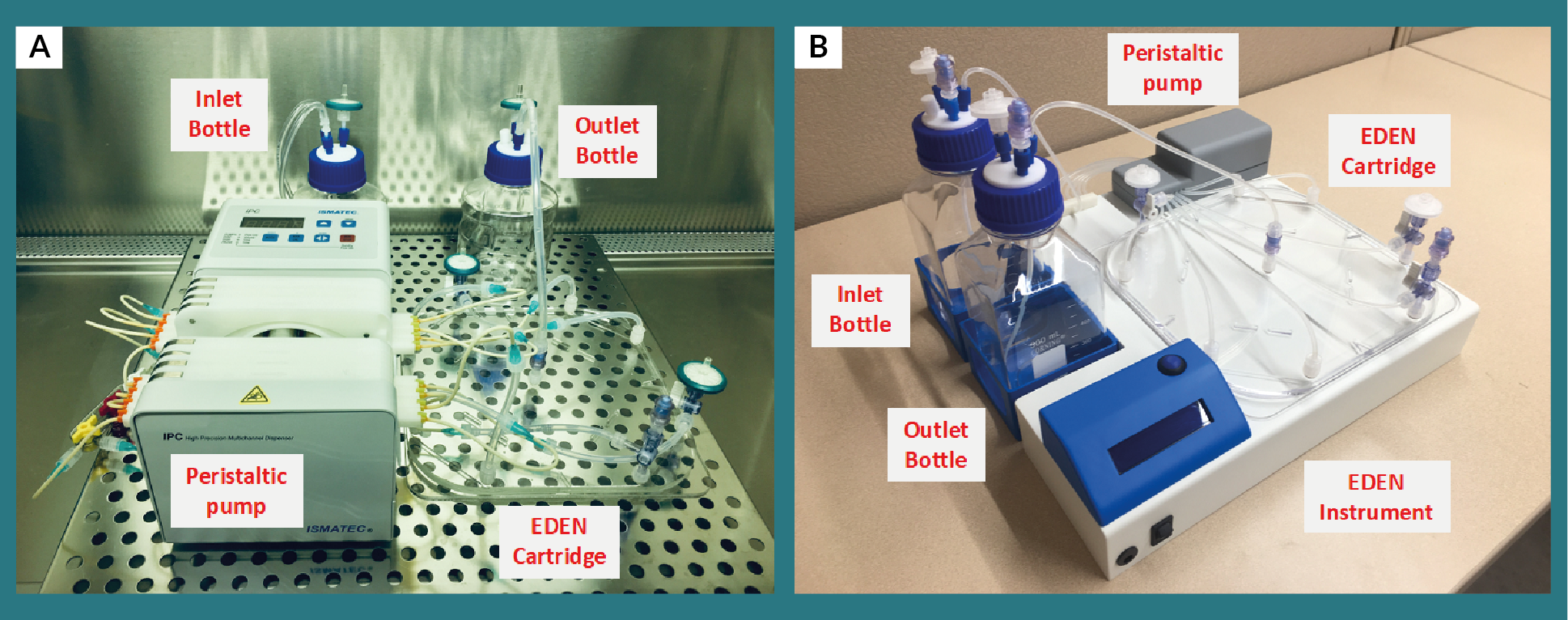
Materials & methods
PBMC isolation & monocyte enrichment
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque (GE Healthcare) from whole blood purchased from StemExpress. The whole blood was drawn and processed on the same day. Isolated PBMCs were cryopreserved at 50–60 million PBMCs/mL in CryoStor CS10 and remained in cryopreservation for at least 7 days prior to resuscitation. Monocytes (MOs) were enriched from resuscitated PBMCs using Miltenyi CD14 MicroBeads and two LS column passes to obtain a MO purity >95%. Enriched MOs from a single donor were suspended in 122 mL differentiation medium and seeded into the EDEN cartridge. Each experiment used MOs from a different donor.
Differentiation medium
RPMI 1640 (Gibco 11875119) was supplemented with 10% heat inactivated-fetal bovine serum (HI-FBS; MilliporeSigma F2442), 1% penicillin-streptomycin (P/S; Gibco 15140122), 500 U/mL IL-4 (R&D Systems 204IL), and 500 U/mL GM-CSF (R&D Systems 215GM).
EDEN cartridge & fluidic system
The EDEN cell culture cartridge was fabricated from commercially available polystyrene and acrylate cut using an Epilog Zing 16 laser system and assembled using 3M 966 Adhesive Transfer Tape. The polystyrene base was plasma treated. The cartridge has an internal surface area of 383.6 cm2, volume of 122 mL, and measures 21.0 cm x 21.0 cm x 0.317 mm (length x width x height). Table 1 shows the number of MOs seeded. Eight inlet ports around the perimeter allow fresh differentiation medium to perfuse into the cartridge and a single outlet port at the center allows depleted medium to be removed from the cartridge.
| Table 1. Differentiation data for iDC generation in EDEN and 6-well plates. | |||||||
|---|---|---|---|---|---|---|---|
| Culture Vessel | MOs seeded (x106) | Seeding density (MOs per cm2) | Cells harvested (x106) | Viable CD45+cells | iDCs CD209+ CD14- | Viable iDCs harvested (x106) | iDC yield |
| EDEN 1 | 114.3 | 300,200 | 26.7 | 98.30% | 94.90% | 24.9 | 21.80% |
| EDEN 2 | 78.3 | 205,700 | 25.8 | 96.20% | 94.90% | 23.6 | 30.10% |
| 6-well plate 1 | 3.48 | 366,000 | 1.17 | 95.40% | 96.80% | 1.08 | 31.00% |
| 6-well plate 2 | 1.74 | 183,000 | 0.47 | 94.10% | 97.80% | 0.43 | 24.70% |
| Phenotype data is shown in Figure 4. | |||||||
The fluidic system consisted of an inlet bottle for fresh differentiation medium, peristaltic pump, and outlet bottle for collecting effluent from the cartridge. An Ismatec IPC-N peristaltic pump was used with PharMED BPT tubing to maintain continuous perfusion of fresh differentiation medium at 8.0 μL/min/inlet. Silicone tubing was connected between the peristaltic tubing and cartridge inlet to facilitate gas exchange between the medium and ambient environment maintained at 37ºC and 5% CO2 inside a Thermo Forma incubator. Silicone tubing was also used at the outlet port where perfusion flow rate was 64 μL/min. Effluent collected in the waste reservoir was centrifuged to determine if cells were washed out of the cartridge due to perfusion; no cells were observed in the effluent indicating that generated iDCs remain inside the cartridge and perfusion flow rate is not high enough to resuspend cells residing at the polystyrene base. 285 mL of fresh differentiation medium was added to the inlet reservoir at startup (Day 0) and Day 3 to maintain perfusion throughout the 6-day differentiation. Cells were harvested by collecting the cell solution and washing the cartridge 2x with cold DPBS. Adherent cells after the two DPBS washes were not collected.
6-well plate control
A Corning Costar 6-well plate (3516) was used as a static control for iDC generation. Each well contained 2.5 mL differentiation medium and empty wells were filled with 3.0 mL DPBS. Table 1 shows the number of MOs seeded. 1 mL fresh differentiation medium was added to each well on Day 3. Cells were harvested by collecting the cell solution and washing each well 2x with cold DPBS. Adherent cells after the two DPBS washes were not collected.
Immature DC maturation
Maturation was conducted on the MicroDEN system at 3.5 μL/min perfusion using a small version MicroDEN cartridge that was 17.4 cm2 and held 5.5 mL maturation medium. Maturation medium consisted of RPMI 1640 supplemented with 10% heat inactivated-fetal bovine serum (HI-FBS; Millipore Sigma F2442), 1% penicillin-streptomycin (P/S; Gibco 15140122), 2 ng/mL IL-1β (BD Biosciences 554602), 1000 U/mL IL-6 (BD Biosciences 550071), 10 ng/mL TNF-α (MilliporeSigma 11088939001), and 1 μg/mL PGE2 (MilliporeSigma P6532). Immature DCs from the EDEN 1 experiment were seeded at 422,200 iDCs/cm2 and allowed to mature for either 1 day or 3 days in an incubator at 37ºC and 5% CO2. The cells were harvested using 2 cold PBS washes as described in [16].
Immunophenotyping
An ACEA Biosciences NovoCyte flow cytometer was used for immunophenotyping of harvested iDCs. Panel A tested viability (LIVE/DEAD Fixable Green Dead Cell Stain; Invitrogen L34970), CD209 (R&D Systems FAB 161P100), CD14 (Abcam ab157312), and CD45 (R&D Systems FAB1430A). Panel B tested CD80 (BD Biosciences 557226), CD83 (BD Biosciences 556855), CD86 (BD Biosciences 561128), and CD45; viability was not included due to limited detection channels. Panel C tested CD80, CD83, CD86, and CD209 (R&D Systems FAB161A). Gates were set using a CD209 isotype control (IgG2b-PE R&D Systems IC0041P and IgG2b-APC R&D Systems IC0041A) and fluorescence-minus-one (FMO) controls.
Flow cytometry gating strategy
Large cells were gated in the SSC-A/FSC-A plot followed by single cells in a FSC-A/FSC-H plot. Panel A: viable/CD45+ cells were gated then CD14/CD209 was plotted to determine MO or iDC percentage. Panel B: lymphocytes were gated on a CD45 histogram. Then CD80/83 and CD80/86 was plotted to determine iDC phenotype. Panel C: DCs were gated on a CD209/80 plot followed by a CD83/86 plot on either the CD209+/80+ or CD209+/80– cells.
Results & discussion
Computational fluid dynamics (CFD) simulations
Computational fluid dynamics (CFD) simulations in COMSOL Multiphysics software were utilized in designing EDEN to understand how medium flows within the cartridge. Water at 37ºC was used to simulate differentiation medium. The cartridge was initially filled with plain water without cytokines. In practice, the cartridge is filled with differentiation medium containing cytokines; however, initially filling the cartridge with plain medium (water) allows cytokine convection to be visualized since cytokine diffusion is extremely low (9216 μm2/day) [17–19] and convection is the driving force behind the cytokine gradient. Water containing 1.16 mol/m3 (500 U/mL) R&D Systems IL-4 was perfused into the cartridge at 8.0 μL/min/inlet and exited through the outlet at the cartridge center. Cytokine consumption/depletion was not factored into this analysis since we were interested in determining optimum medium flow of fresh differentiation medium. Figure 2A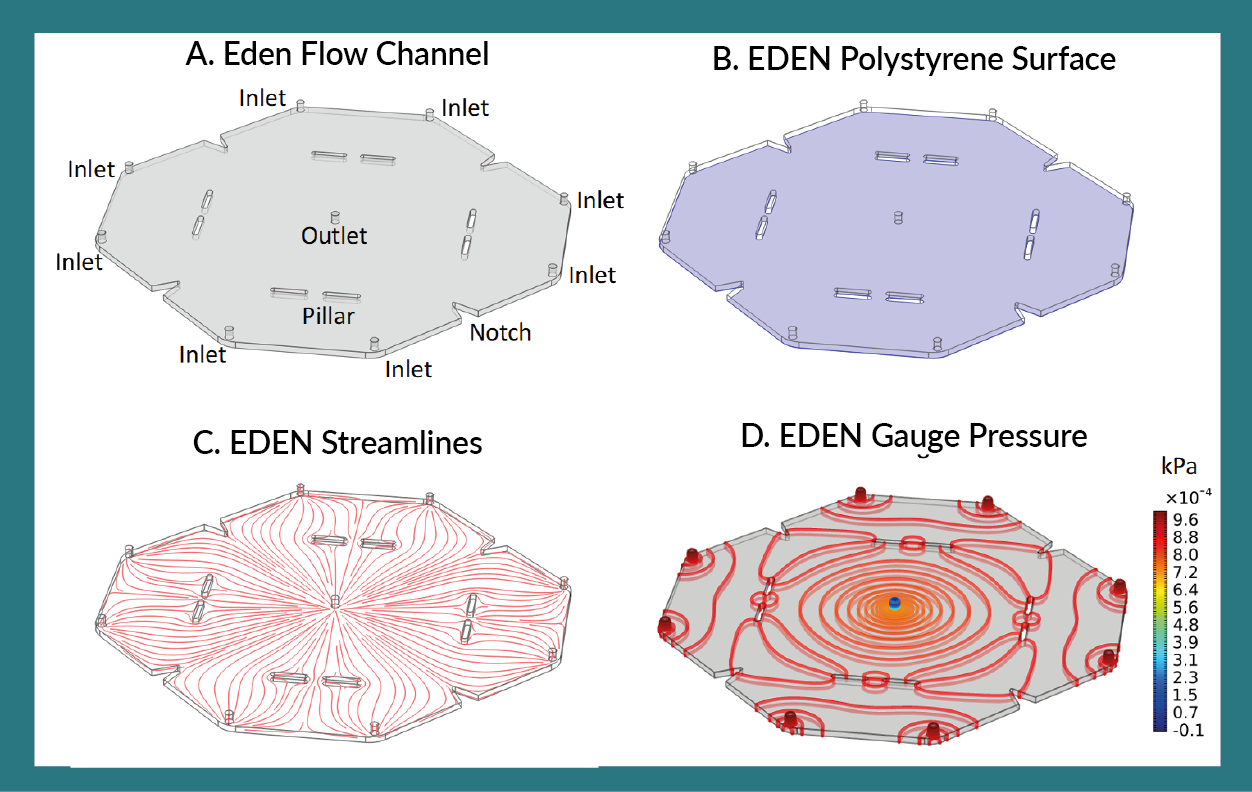
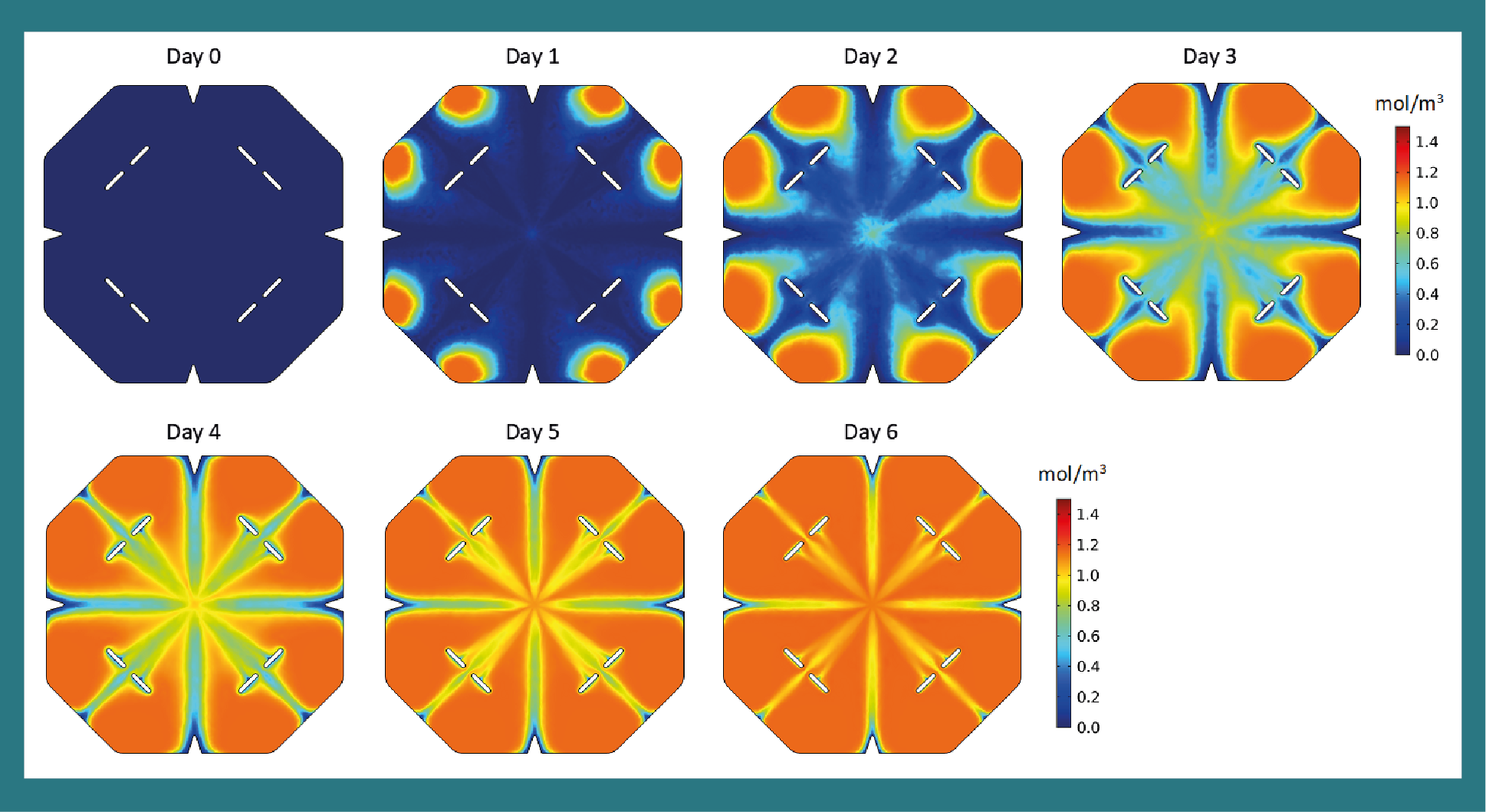
These CFD data were critical in designing a cartridge which sufficiently allowed perfused medium to spread throughout the cartridge. Cytokine concentration and streamline data shows that at 8.0 μL/min/inlet laminar flow, the cartridge is split between eight regions. Each region is replenished with fresh differentiation medium after ca. 4 days. Initial CFD simulations indicated that dead zones formed at the location of the v-shaped notches, thus these notches were added to eliminate the dead zones and facilitate desired fluid flow. The eight cylindrical pillars within the cartridge support the upper acrylic surface. Before these were added, slight sagging of the acrylic was observed and the acrylic was supported by medium within the cartridge which would cause unnecessary pressure within the cartridge that may affect the cells. Thus, these features, i.e., the notches and pillars, were added to alleviate the dead zone and pressure concerns resulting in the final EDEN cartridge design that sufficiently aided perfused medium to flow within the cartridge without causing undesired pressure gradients.
Immature DC generation
Two iDC generation experiments were conducted in which 114.3 million and 78.3 million MOs were seeded into the EDEN cartridge. After 6 days differentiation, 24.9 million and 23.6 million iDCs were harvested from each cartridge. The number of viable iDCs harvested was calculated by multiplying total cells harvested by viable/CD45+ cells by iDCs (CD209+/14–). IDC yield (normalized to the number of MOs seeded) was calculated as the number of iDCs harvested divided by the MOs seeded and was 21.8% and 30.1% for the two EDEN experiments. 6-well plate controls show that iDC yield was similar to EDEN, where the well plate had a higher yield than EDEN in experiment 1 and a lower yield in experiment 2. Titration of MO seeding density is necessary to optimize iDC yield in EDEN. Tabulated data are shown in Table 1.
Immature DC phenotype
Immunophenotyping of generated iDCs are shown in Figure 4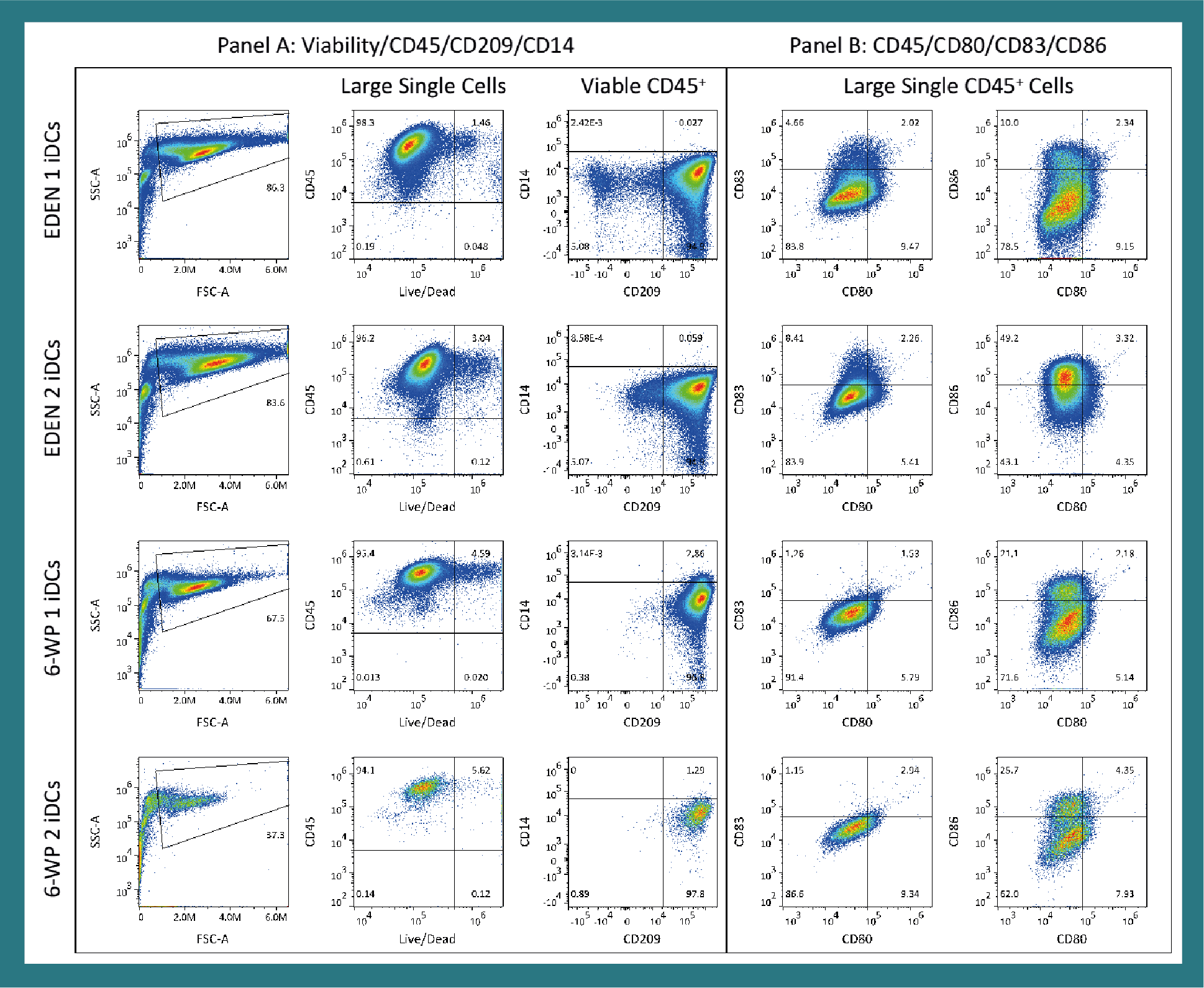
Immature DC maturation
Immature DCs generated in EDEN 1 were subsequently matured in a MicroDEN cartridge for either 1 day or 3 days. 7.31 million iDCs were seeded into each MicroDEN cartridge (422,200 iDCs/cm2) and 5.7 million (1 day maturation) and 3.4 million (3 day maturation) mature DCs (mDCs) were harvested, for a yield of 77.8% and 46.5%, respectively. Yield was calculated as the number of seeded iDCs divided by the number of harvested mDCs. Maturation results are tabulated in Table 2 and immunophenotype is shown in Figure 5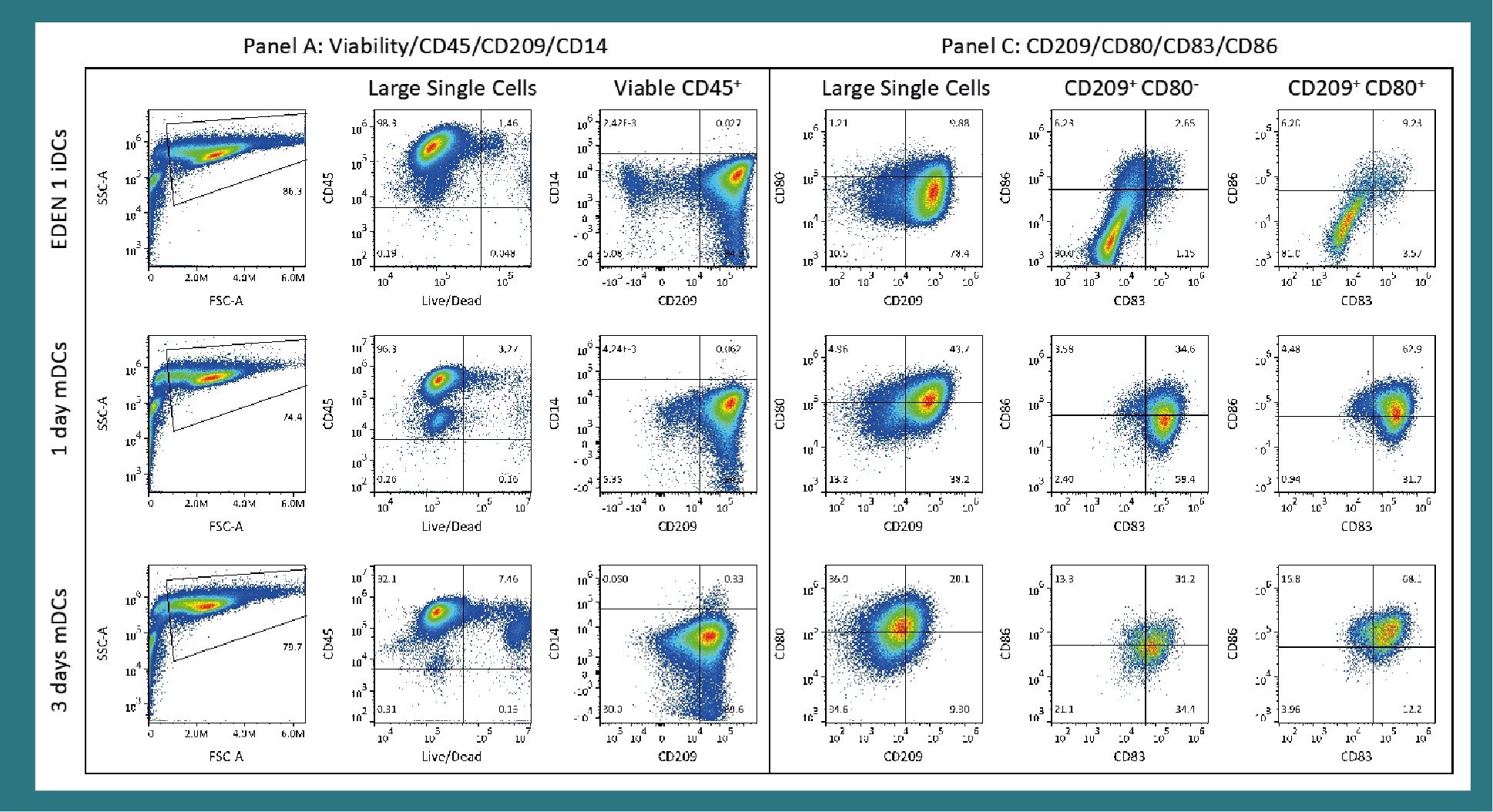
| Table 2. Maturation data for EDEN 1 generated iDCs. | |||||||
|---|---|---|---|---|---|---|---|
| Experiment | iDCs seeded (x106) | Seeding density (iDCs per cm2) | Cells harvested (x106) | Viable CD45+cells | mDCs CD209+ CD14- | Viable mDCs harvested (x106) | mDC yield |
| 1-day maturation | 7.31 | 422,200 | 6.24 | 96.30% | 94.60% | 5.7 | 77.80% |
| 3-day maturation | 7.31 | 422,200 | 5.3 | 92.10% | 69.60% | 3.4 | 46.50% |
| Maturation was performed in a small version MicroDEN cartridge. Phenotype data is shown in Figure 5. | |||||||
Interestingly, both CD209 markers (Panel A and Panel C) showed a slight decrease of CD209 expression after 1-day maturation of iDCs and a significant decrease of CD209 expression after 3 days maturation. The low mature DC (mDC) yield for 3-day maturation was due to the decrease of CD209 expression of these cells. The CD209– population decreased from ca. 5% for iDCs and 1-day matured mDCs to 30% for 3-day matured DCs. CD80 expression increased from ca. 10% for iDCs to 44% for 1-day maturation; whereas, 3-day maturation yielded 56% CD80+ cells of which only 20% were also CD209+. CD80 expression is generally low on iDCs and upregulated on mDCs, indicating successful maturation [20–22,25]. Both CD80+ and CD80– mDCs strongly expressed CD83 (>90%) after 1-day maturation. After 3 days maturation, CD83 was expressed on 80% of CD80+ mDCs but only 65% of CD80– mDC. CD86 expression was greatest for CD80+ mDCs for both 1- and 3-day maturation with 68% and 84% of the mDCs expressing CD86. CD80– mDCs significantly lower level of CD86 expression, ca. 40% for both 1- and 3-day maturation. Collectively, these results indicate that maturation duration significantly affects phenotype and yield of mDCs for the experimental conditions studied. We caution against concluding that 1-day maturation is optimal since function of matured DCs and cytokine excretion should be evaluated in a mixed lymphocyte assay (MLA).
Maturation of iDCs can take place in the same EDEN cartridge as MO differentiation by perfusing maturation medium into the cartridge; however, we decided to harvest iDCs from the EDEN cartridge to allow for counting and immunophenotyping of generated iDCs. One option is to perfuse maturation medium directly into the EDEN cartridge without harvesting the iDCs. A second option is to harvest the iDCs, resuspend in maturation medium, and reseed into the EDEN cartridge. A third option, the path we chose for this work, is to harvest generated iDCs from the EDEN cartridge, resuspend in maturation medium after counting cells and removing 2 million DCs for phenotyping, and seeding into a smaller MicroDEN cartridge for 1 or 3 days maturation while perfusing maturation medium. The desired workflow will depend on user requirements, e.g., obtaining iDC cell count, tailoring maturation cocktail to the number of iDCs, and iDC concentration/seeding density for maturation.
Production of therapeutically active DCs in EDEN
Bespoke production of therapeutically active DCs follows the same general outline regardless of the targeted disease. The exact protocol will depend upon the desired characteristics of the DCs and EDEN is designed to be easily integrated into current vaccine production protocols. The MicroDEN can be used to optimize differentiation, maturation, and peptide pulsing conditions before advancing to larger-scale therapeutically active DC generation in EDEN. It is recognized that monocyte enrichment (e.g., via elutriation or magnetic beads) must precede DC generation in EDEN and some manual handling is required to perform the DC generation process in EDEN; however, with sterile transfer-compatible consumable design, the entire process can be carried out in a closed format.
The following is an example workflow incorporating EDEN for generating therapeutic DCs. Figure 6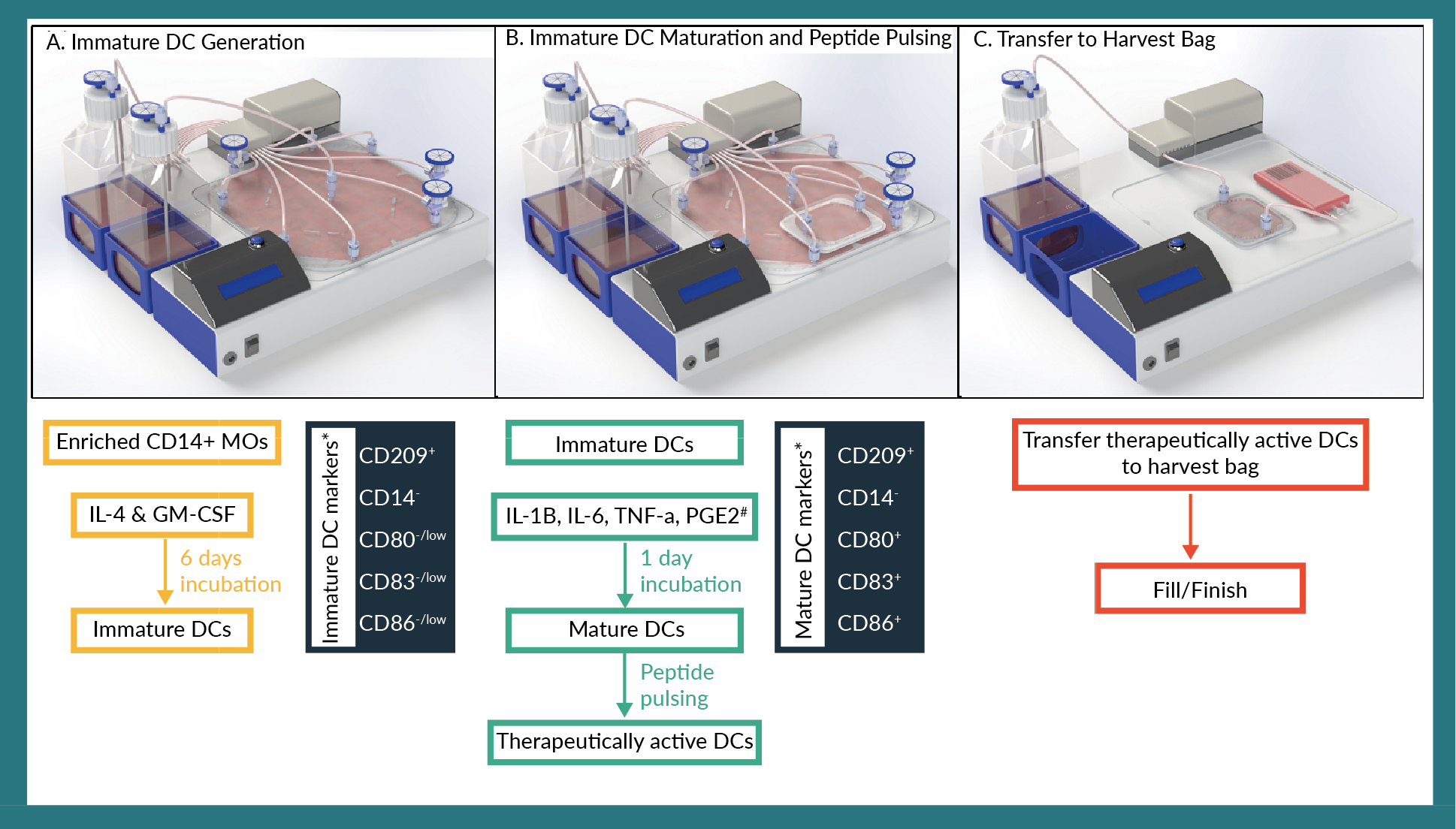
- CD14+ MOs are cultured with IL-4 and GM-CSF for 5–10 days to generate iDCs. These iDCs will be CD14– and CD209 (DC-SIGN)+. Few cells will express CD80/83 while a greater number of cells will be CD86+;
- Immature DCs are either (a) harvested from EDEN, resuspended in maturation medium, and seeded back into the EDEN cartridge or a MicroDEN cartridge depending on the desired cell concentration during maturation or (b) left within the EDEN cartridge (i.e., not harvested) and maturation medium is perfused into the cartridge;
- Immature DCs are matured for typically 1–2 days in maturation medium followed by peptide pulsing of typically 2–24 hours [8,13,26]. The mDCs express CD80/83/86 and have lower expression of CD209 compared to iDCs. Once the mDCs are pulsed with the target peptide(s), the DCs are considered therapeutically active. Figure 6B depicts cells being transferred from an EDEN cartridge to a MicroDEN cartridge for maturation and peptide pulsing;
- The therapeutically active DCs are transferred from either the MicroDEN cartridge (Figure 6C) or the EDEN cartridge into a harvest bag.
Conclusions
EDEN was developed for GMP production of therapeutically relevant numbers of iDCs in a single cell culture cartridge that is fully enclosed and unopen to the outside environment. Computational fluid dynamics simulations aided the design of the EDEN cartridge to ensure that perfused medium flowed properly throughout the cartridge and cytokines were sufficiently replenished. Fresh differentiation medium was continuously perfused into the cartridge and depleted medium was concomitantly removed. Phenotype expression and yield of MO-iDCs was similar between EDEN and 6-well plate controls. Immature DCs were subsequently matured in a MicroDEN cartridge and exhibited standard upregulation of CD80/83/86 and downregulation of CD209. These results show that EDEN successfully generates 20–25 million iDCs with a 20-30% iDC yield at the conditions tested and demonstrate that EDEN is a viable option for scaling-up GMP production of therapeutically active dendritic cells.
ACKNOWLEDGEMENT
This material is based upon work supported by the U.S. National Science Foundation under Grant No. 1819306.
FINANCIAL & COMPETING INTERESTS DISCLOSURE
AK is an employee and shareholder, HW is an employee, SKM is a founder and shareholder, and JMR is President & CEO and a shareholder of Flaskworks LLC which is commercializing the EDEN system. No writing assistance was utilized in the production of this manuscript.
REFERENCES
1. Roh KH, Nerem RM, Roy K. Biomanufacturing of therapeutic cells: State of the art, current challenges, and future perspectives. Annu. Rev. Chem. Biomol. Eng. 2016; 7: 455–78. CrossRef
2. Tuyaerts S, Aerts JL, Corthals J et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol. Immunother. 2007; 56: 1513–37. CrossRef
3. Babatz J, Röllig C, Oelschlägel U et al. Large-scale immunomagnetic selection of CD14+ monocytes to generate dendritic cells for cancer immunotherapy: A phase I study. J. Hematother. Stem Cell Res. 2003; 12: 515. CrossRef
4. Dietz AB, Padley D, Butler G et al. Clinical-grade manufacturing of DC from CD14(+) precursors: Experience from phase I clinical trials in cml and malignant melanoma. Cytotherapy 2004; 6: 563–70. CrossRef
5. Felzmann T, Witt V, Wimmer D et al. Monocyte enrichment from leukapharesis products for the generation of DCs by plastic adherence, or by positive or negative selection. Cytotherapy 2003; 5: 391. CrossRef
6. Garlie N, Timler A. Dendritic cell generation from cryopreserved monocytes enriched using the Elutra versus the CliniMACS cell separation systems. J. Immunother. 2005; 28: 613. CrossRef
7. Meyer-Wentrup F, Burdach S. Efficacy of dendritic cell generation for clinical use: Recovery and purity of monocytes and mature dendritic cells after immunomagnetic sorting or adherence selection of CD14+ starting populations. J. Hematother. Stem Cell Res. 2003; 12: 289. CrossRef
8. Carreno BM, Magrini V, Becker-Hapak M et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015; 348: 803–8. CrossRef
9. Carreno BM, Becker-Hapak M, Huang A et al. IL-12p70–producing patient DC vaccine elicits Tc1-polarized immunity. J. Clin. Invest. 2013; 123: 3383–94. CrossRef
10. Linette GP, Zhang D, Hodi FS et al. Immunization using autologous dendritic cells pulsed with the melanoma-associated antigen gp100-derived G280-9V peptide elicits CD8+ immunity. Clin. Cancer Res. 2005; 11: 7692. CrossRef
11. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994; 179: 1109. CrossRef
12. Mitchell DA, Batich KA, Gunn MD et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015; 519: 366. CrossRef
13. Nair S, Archer GE, Tedder TF. Isolation and generation of human dendritic cells. Current Protocols in Immunology Chapter 7: Unit7.32-Unit37.32; 2012. CrossRef
14. Garg AD, Vara Perez M, Schaaf M et al. Trial watch: Dendritic cell-based anticancer immunotherapy. OncoImmunology 2017; 6: e1328341. CrossRef
15. Saxena M, Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer 2018; 4: 119–37. CrossRef
16. Kozbial A, Bhandary L, Collier BB, Eickhoff CS, Hoft DF, Murthy SK. Automated generation of immature dendritic cells in a single-use system. J. Immunol. Methods 2018; 457: 53–65. CrossRef
17. Gammack D, Doering CR, Kirschner DE. Macrophage response to mycobacteriumtuberculosis infection. J. Math. Biol. 2004; 48: 218–42. CrossRef
18. Pigozzo AB, Macedo GC, dos Santos RW, Lobosco M. On the computational modeling of the innate immune system. BMC Bioinformatics 2013; 14: S7–S7. CrossRef
19. Su B, Zhou W, Dorman KS, Jones DE. Mathematical modelling of immune response in tissues. Comput. Math. Methods Med. 2009; 10. CrossRef
20. Mo-DC generation toolbox: https://www.miltenyibiotec.com/_Resources/Persistent/898185d33bc556eb522f23f625388dfa0729cd36/DS_130-093-568.pdf
21. Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology 2013; 140: 22–30. CrossRef
22. Dudek A, Martin S, Garg A, Agostinis P. Immature, semi-mature, and fully mature dendritic cells: Toward a DC-cancer cells interface that augments anticancer immunity. Front. Immunol. 2013; 4: 438. CrossRef
23. Zhang R, Becnel L Li M, Chen C, Yao Q. C-reactive protein impairs human CD14+ monocyte-derived dendritic cell differentiation, maturation and function. Eur. J. Immunol. 2006; 36: 2993–3006. CrossRef
24. Sansom DM, Manzotti CN, Zheng Y. What’s the difference between CD80 and CD86? Trends Immunol. 2003; 24: 313–8. CrossRef
25. Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods 1996; 196: 121–35. CrossRef
26. Shinde P, Fernandes S, Melinkeri S, Kale V, Limaye L. Compromised functionality of monocyte-derived dendritic cells in multiple myeloma patients may limit their use in cancer immunotherapy. Scientific Rep. 2018; 8: 5705. CrossRef
AFFILIATIONS
Andrew Kozbial1, Hope Weinstein1, Shashi K Murthy2 & Jennifer M Rossi1
1Flaskworks LLC, Boston MA 02118, USA
2Northeastern University, Department of Chemical Engineering, Boston MA 02115, USA