Why Pall’s Allegro™ Stirred-Tank Bioreactor is ideal for viral vector cell culture
Cell & Gene Therapy Insights 2022; 8(7), 781–789
DOI: 10.18609/cgti.2022.118
Viral vectors facilitate the delivery of genetic material to living cells for the potential treatment of multiple genetic diseases. With recent regulatory approvals, the rapid growth in demand for viral vector-based products highlights the need for proven, scalable manufacturing solutions that can fully meet this demand and ultimately increase the availability of viral vector-based treatments. Pall’s AllegroTM STR stirred-tank bioreactor addresses the need for scalability, as it can be scaled up to 2000 L to enable the manufacture of viral vectors. In this article, we discuss the key attributes of the Allegro STR bioreactor such as the design, scalability, agitation and sparging which make it ideal for viral vector manufacture at a larger scale. Also, we show process scalability under controlled key parameters from Allegro STR 50 L to 500 L based on cell growth, metabolic profile, and viral vector production.
Gene therapy has made significant advances over the past two decades. Gene transfer therapy involves the administration of specific genetic material (i.e., DNA or RNA) via a carrier, known as a ‘vector’. Viral vectors offer a new class of biologics which facilitates gene transfer and modification in living cells, potentially treating many conditions with genetic causes. Currently, the most used viral vectors for gene transfer therapy include gamma retrovirus (RV), adenovirus (AV), adeno-associated virus (AAV), and lentivirus (LV) [1]Sayed N, Allawadhi P, Khurana A, Singh V et al. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022; 294(2022), 120375. .
Previously, gene therapy mainly addressed rare or very rare diseases and therefore the manufacture of gene therapy viral vectors were only set out to meet the market demands of a relatively small group of patients within the orphan disease market space, where meeting demand has not always been a big problem. Advancement in gene transfer therapy-based treatments and its inevitable extension to common indications such as cancer, Parkinson’s and Alzheimer’s means that gene therapy viral vectors must be manufactured in larger scales. This production gap is one of the main challenges in the gene transfer therapy field.
According to Precedence Research, the global gene therapy market is expected to be valued at over US$15 billion by 2030 [2]Gene Therapy Market Size, Growth, Trends, Report 2021–2030. . This expected growth has generated a huge pressure on biomanufacturing companies to develop new technologies to be able to satisfy the high demand of gene therapy products / technologies.
This trend is also driving a greater need for the scalable production of viral vectors for gene therapy. Traditionally, viral vector production is mostly based on adherent cell lines using systems such as multi-trays, that can only be scaled out. Adherent bioreactors such as Pall’s iCELLis® bioreactor have been developed during the past decade allowing to scale up of such processes up to a certain surface (500 m2 for the iCELLis 500+ bioreactor). Over recent years, more manufacturers have investigated adapting their cells for viral vector manufacturing to suspension culture to reach higher volume vector-producing batches, that can be required for large dose/large population applications. Pall developed the Allegro Stirred-Tank (STR) Bioreactor for suspension cells which can be easily scaled up to 2000 L (Figure 1 Pall Allegro STR 50, 200, 500, 1000 and 2000 L bioreactors.). Pall’s expertise and understanding of process scaling technology has enabled large scale manufacturing of gene therapy products to meet the ever-increasing demand.
Pall Allegro STR 50, 200, 500, 1000 and 2000 L bioreactors.). Pall’s expertise and understanding of process scaling technology has enabled large scale manufacturing of gene therapy products to meet the ever-increasing demand.
In Pall’s Allegro STR bioreactor, cell growth is substrate independent, hence high viable cell densities can be achieved and more importantly, these cells can produce high titers of viral vectors, including AAV, LV and AV. The Allegro STR bioreactors provide optimal environment for various cell types to reach their full growth and viral productivity potential.
Design of Pall Allegro STR bioreactor
The success of meeting the increasing demand of gene therapy heavily depends on the provision of more bioreactor manufacturing capacity. The COVID-19 pandemic has added to the strained capacity as some of the vaccine’s programs are also using viral vectors. Pall’s Allegro STR bioreactors are perfect candidates to reduce this capacity crunch in producing viral vectors for gene transfer therapy.
The Allegro STR bioreactor family combines Pall’s bioprocess engineering expertise, cell culture know-how and our drive for quality into a series of single-use bioreactors that deliver consistent and scalable cell culture performance for cell culture and viral vector production across the Allegro STR bioreactor range. From the outset of the design, Pall placed strong emphasis on providing compact, ergonomic, and intuitive turnkey bioreactor design concepts to maximize usability and process assurance, while maintaining optimal performance and reliability needed in a cell culture and viral vector production environment through several easy and intuitive operation features such as [3]Nienow AW, Isailovic B, Barrett TA et al. Design and Performance of Single-Use, Stirred-Tank Bioreactors. BioProcess Int. 2016; 14(10), 12–21.:
- A bottom mounted pitched blade ‘elephant ear’ impeller with three 45-degree angle blades to promote efficient axial and radial mixing in a cuboid-shaped bioreactor (unique to Pall’s Allegro STR bioreactors), while supporting options for both upward and downward flow depending on the application required. This type is common in bioreactors used for animal-cell culture because it is considered less likely to cause shear damage with optimal blade diameters and agitation speeds while ensuring effective mixing and oxygen mass transfer for high cell density cell
culture [3,7]; - Wide range of agitation power inputs (W/m3) for efficient mixing and gas dispersion;
- Headspace volume at ~25%, providing adequate allowance for high hold-up (and possible foaming) associated with high specific power and aeration rates;
- Three baffles that eliminate the need for customized shaping and welding of flexible side walls during manufacture and maximize biocontainer strength, integrity, and robustness;
- A cubical biocontainer with aspect ratio H/T = 1 has a similar volume to the cylindrical format with a ratio >1 (Figure 2
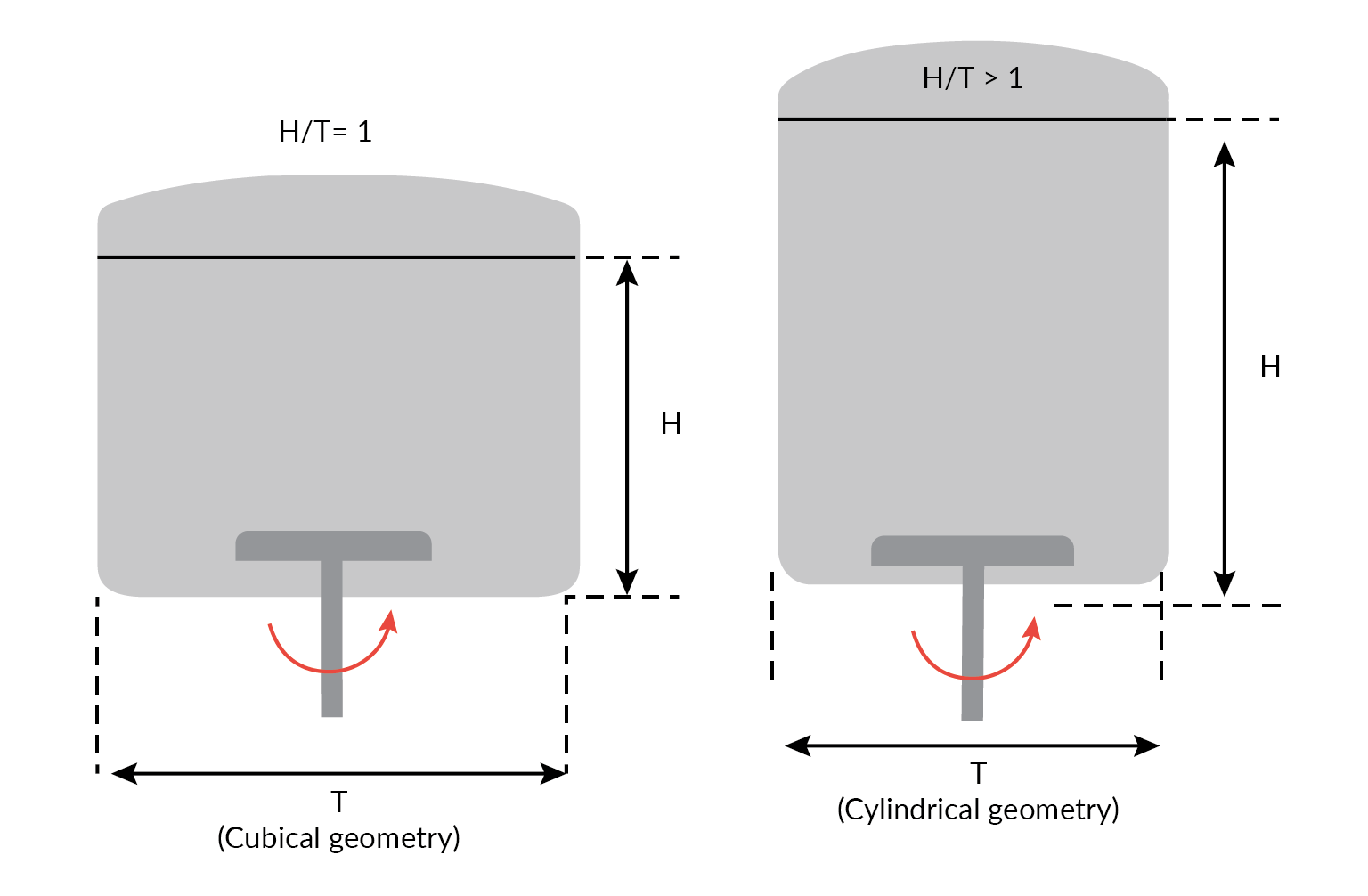 Aspect ratio of a square cross-section Allegro STR bioreactor compared with a cylindrical bioreactor of similar volume). Because aspect ratios >1 can lead to poor homogeneity at the top surface, the cubical format’s lower aspect ratio with its reduced fluid height provides for improved mixing and a greater headspace mass transfer capacity. This can allow for minimal sparging and enhanced CO2 stripping;
Aspect ratio of a square cross-section Allegro STR bioreactor compared with a cylindrical bioreactor of similar volume). Because aspect ratios >1 can lead to poor homogeneity at the top surface, the cubical format’s lower aspect ratio with its reduced fluid height provides for improved mixing and a greater headspace mass transfer capacity. This can allow for minimal sparging and enhanced CO2 stripping; - Use of computation fluid dynamics modeling studies to ensure that cuboid shaped bio-container matches those of conventional cylindrical stainless-steel bioreactors [3], performance further verified by empirical studies;
- Installation and inflation of the biocontainer is achieved in <30 minutes through a guided sequence via the Human Machine Interface (HMI) for ease-of-use;
- All product contact surfaces in the Allegro STR bioreactors are single-use components that are cell culture compatible, thus reducing the demands and cost of maintenance, cleaning, and cleaning validation to a minimum;
- Addressing footprint restrictions in cleanrooms: With a maximum height of 2.9 meters for the 2000 L unit-scale STR, Pall’s Allegro STR bioreactors are compact and are easily accommodated and installed into laboratories and manufacturing sites, negating the need for extensive re-fitting and installation associated costs such as hoists and ladders;
- See Nienow, Isalovic and Barret, 2016 [3] for further details on the bioreactor design considerations that were optimized during the design of the Pall’s Allegro STR bioreactors.
Scalability
Scalable manufacturing is one of the critical processes required to be able to provide the quantities of vector needed to bring these potentially life-saving treatments to waiting patient populations. Many gene therapy manufacturing processes rely on culturing HEK293 cell lines (or derivative AAV293 cell lines), and several early and current forms of production culture these cells on an adherent substrate [4]Sanderson T, Erlandson T, Hazi N et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell Gene Ther. Ins. 2021; 7(9), 1025–1033.
Pall’s expert knowledge of process scaling, and critical scaling parameters ensures that processes are easily scaled up or down across all sizes of the Allegro STR bioreactors with working volumes ranging from 10 to 2000 L with focus on critical scaling parameters such as specific power input, kLa (volumetric oxygen mass transfer coefficient), mixing time, and aspect ratio so that cell culture environment and conditions are as similar as possible regardless of size [4]Sanderson T, Erlandson T, Hazi N et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell Gene Ther. Ins. 2021; 7(9), 1025–1033.
In a scaling study using Allegro STR 50 and 500 bioreactors for production of rAAV viral vector published by Sanderson et al., productivity between the two scales was compared [4]Sanderson T, Erlandson T, Hazi N et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell Gene Ther. Ins. 2021; 7(9), 1025–1033. Both STRs were inoculated from the same cell culture bolus at half capacity and expanded to the full working volume after 24 hours. The operational parameters were matched throughout the process. The results showed near identical cell growth and viability between both the Allegro STR 50 and STR 500 bioreactor cultures up until transfection on day 3. After transfection, there was a drop in viability in the two STRs while the viable cell density continued to increase. Both cultures reached a maximum viable cell density of ∼1.8 x 106 cells/mL (Figure 3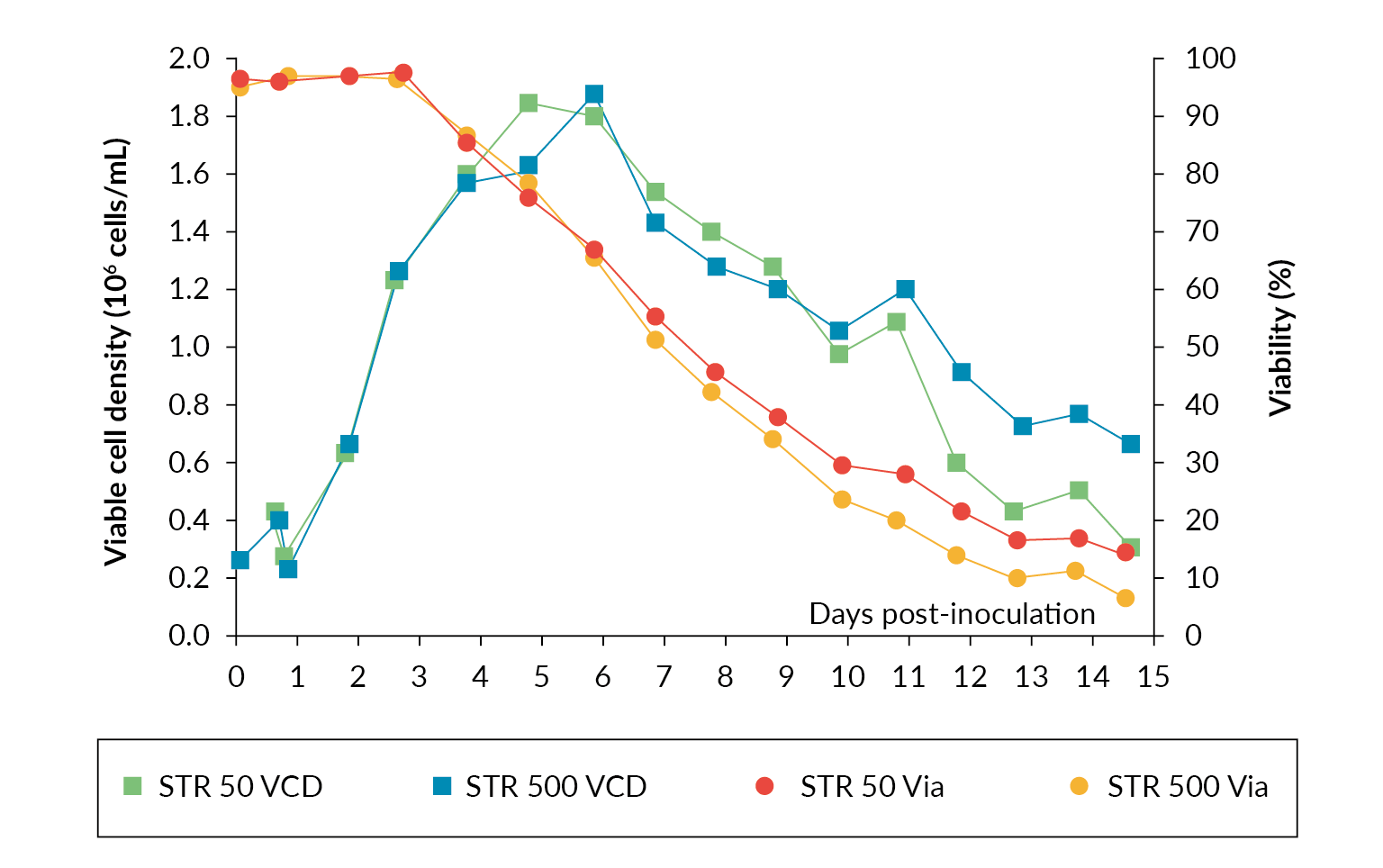 Cell growth and viability profiles in Allegro STR 50 and STR 500 bioreactors.). The data shows rAAV titer increases throughout the culture with maximum titer being observed at harvest. The final rAAV titers were 4.3 x 1010 gc/mL and 4.8 x 1010 gc/mL for the Allegro STR 500 and STR 50 bioreactors respectively (Figure 4
Cell growth and viability profiles in Allegro STR 50 and STR 500 bioreactors.). The data shows rAAV titer increases throughout the culture with maximum titer being observed at harvest. The final rAAV titers were 4.3 x 1010 gc/mL and 4.8 x 1010 gc/mL for the Allegro STR 500 and STR 50 bioreactors respectively (Figure 4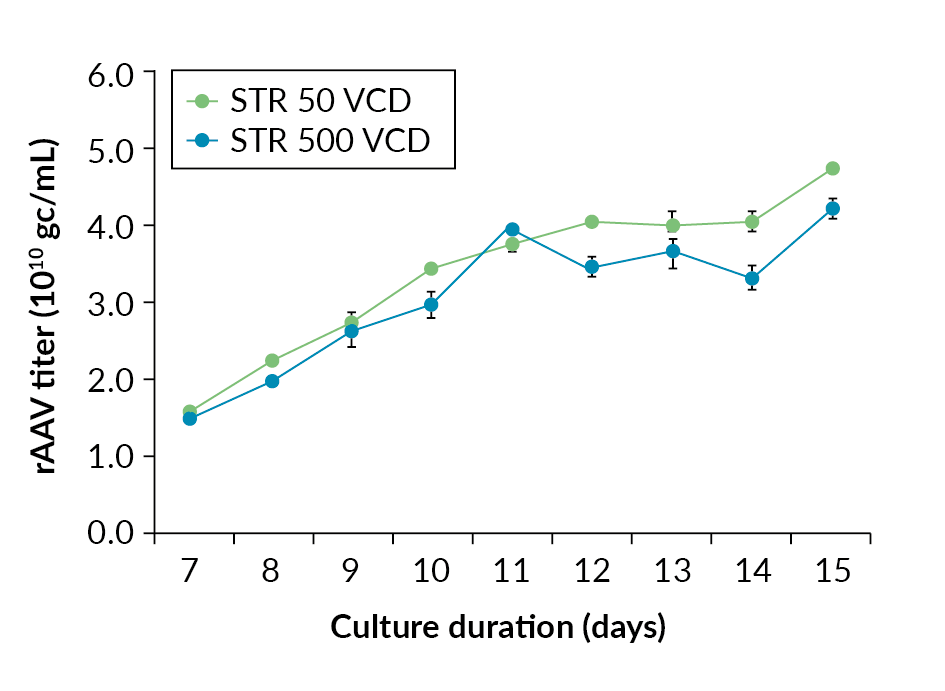 rAAV titers in gc/mL in Allegro STR 50 and STR 500 bioreactors.), comparable in range to those reported in the literature [5]Chahal PS, Schulze E, Tran R, Montes J, Kamen AA. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods 2014; 196: 163–73. , [6]Trivedi PD, Yu C, Chaudhuri P et al. Comparison of highly pure rAAV9 vector stocks produced in suspension by PEI transfection or HSV infection reveals striking quantitative and qualitative differences. Mol. Ther. Methods Clin. Dev. 2021; 24: 154–170. .
rAAV titers in gc/mL in Allegro STR 50 and STR 500 bioreactors.), comparable in range to those reported in the literature [5]Chahal PS, Schulze E, Tran R, Montes J, Kamen AA. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods 2014; 196: 163–73. , [6]Trivedi PD, Yu C, Chaudhuri P et al. Comparison of highly pure rAAV9 vector stocks produced in suspension by PEI transfection or HSV infection reveals striking quantitative and qualitative differences. Mol. Ther. Methods Clin. Dev. 2021; 24: 154–170. .
The nutrient and metabolites were also analyzed daily throughout the production run, and they were comparable. Figure 5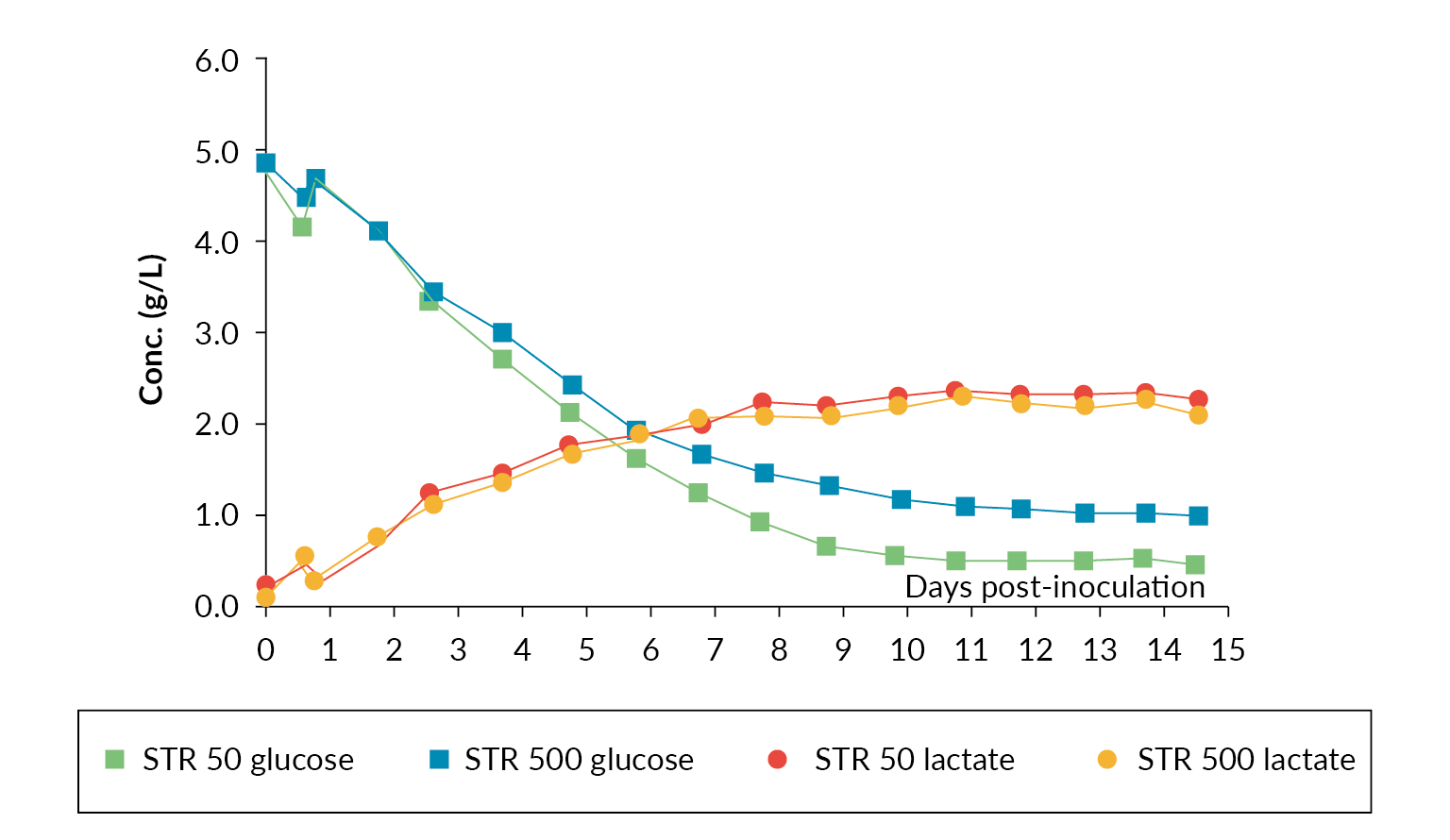 Glucose and lactate profiles in Allegro STR 50 and STR 500 bioreactors. shows a comparison of the glucose and lactate measurements.
Glucose and lactate profiles in Allegro STR 50 and STR 500 bioreactors. shows a comparison of the glucose and lactate measurements.
The result from this comparative study demonstrates that the Allegro STR 50 and STR 500 bioreactors are appropriate for rAAV production and that they provide similar bioreactor cell culture conditions at both the 50 and 500 L scales. This scalability is realized when utilizing Pall’s recommended scale up strategy across the Allegro STR family [4]Sanderson T, Erlandson T, Hazi N et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell Gene Ther. Ins. 2021; 7(9), 1025–1033.
Agitation
Cell damage caused by agitation is a topic that is commonly discussed in the industry but the design features of the Allegro STR bioreactor are such that they mitigate damage from shear. As discussed previously [3]Nienow AW, Isailovic B, Barrett TA et al. Design and Performance of Single-Use, Stirred-Tank Bioreactors. BioProcess Int. 2016; 14(10), 12–21., a modern theory for damage to a range of cell types, including those on microcarriers, suggests that it occurs when cells are larger than the Kolmogorov eddy size, λK:
λK = (ν3 / ΦεT)1/4
where ν is the kinematic viscosity, Φ is the ratio of the maximum local energy dissipation rate compared to the average, and εT is the specific power input in W/kg (1 W/kg = ~103 W/m3 for fluids of a density similar to cell culture media). In the case of the Allegro STR bioreactors, Φ is ~15 [3]Nienow AW, Isailovic B, Barrett TA et al. Design and Performance of Single-Use, Stirred-Tank Bioreactors. BioProcess Int. 2016; 14(10), 12–21.. To avoid cell damage, clearly λK must be >~20 µm (average HEK293 cell size). However, at the maximum speed available, εT = 0.4 W/kg and λK = ~35 µm. Thus, cell damage should not occur [7]Nienow AW. Reactor Engineering in Large Scale Animal Cell Culture. Cytotechnology 2006; 50, 9–33..
For most animal cell cultures, the Allegro STR bioreactors would be programmed for up-flow pumping, even if the system can do both directions. The Allegro STR 200 bioreactor impeller drive system is designed for agitation speeds of up to 150 rpm. In the qualification studies, this bioreactor achieved a specific power output of 0.31 W/kg at 150 rpm in the up-flow mode. This specific power level is significantly higher than what is used generally to meet the mass-transfer requirements of currently achievable cell densities [8]USD 2980. Application Note: Characterization and Engineering Performance of the Allegro™ STR 200 Single-Use Stirred Tank Bioreactor System. Pall Corporation: East Hills, NY, 2014. The ratio of impeller diameter to bioreactor side length (D/T) is an important parameter (Figure 6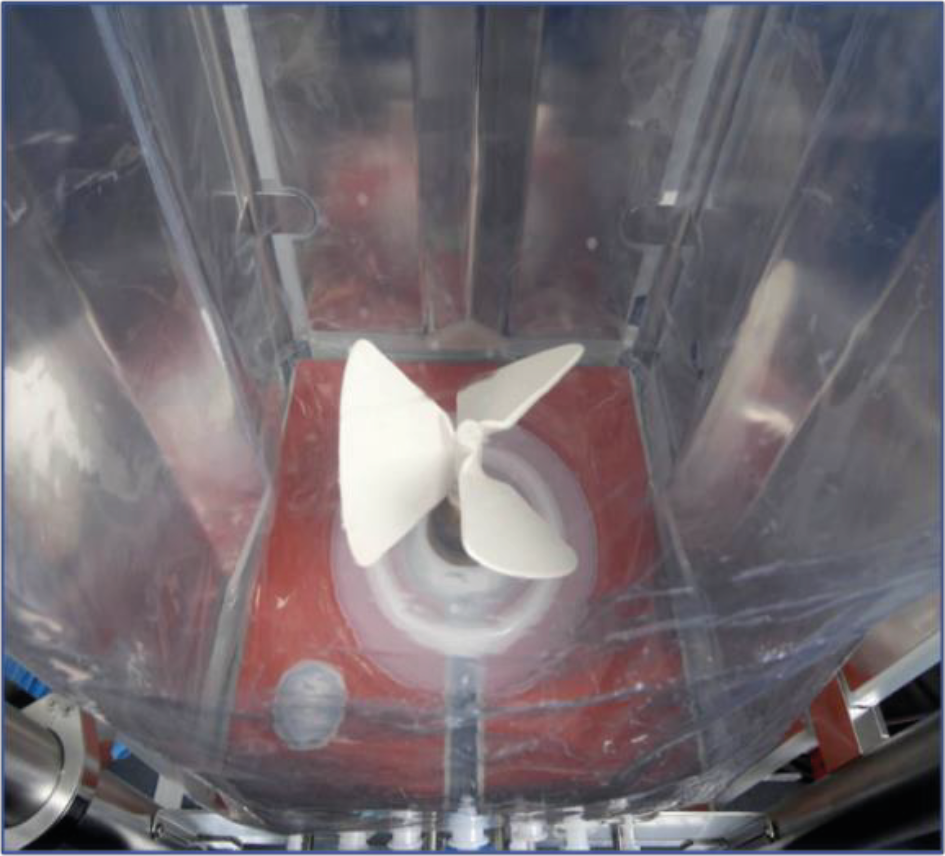 Allegro STR Impeller. & Figure 7
Allegro STR Impeller. & Figure 7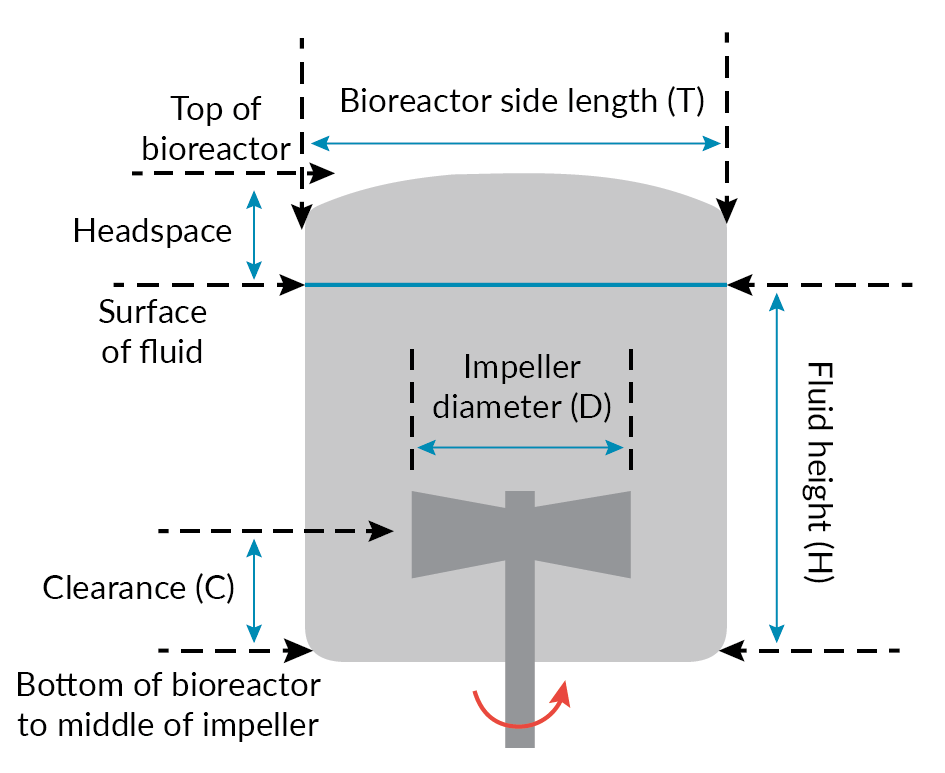 Dimensional representation – Allegro STR bioreactor.) that affects both flow pattern and power input. For the 200 L Allegro STR systems, the D/T ratio was set to 0.5, which for a given specific power input (W/kg) ensures that mixing times are shorter than for smaller impellers [7]Nienow AW. Reactor Engineering in Large Scale Animal Cell Culture. Cytotechnology 2006; 50, 9–33., [9]Isailovic B, Kradolfer M, Rees B. Fluid Dynamics of a Single-Use Stirred Tank Bioreactor for Mammalian Cell Culture. BioProcess Int. 2015; 13(8), 60–66..
Dimensional representation – Allegro STR bioreactor.) that affects both flow pattern and power input. For the 200 L Allegro STR systems, the D/T ratio was set to 0.5, which for a given specific power input (W/kg) ensures that mixing times are shorter than for smaller impellers [7]Nienow AW. Reactor Engineering in Large Scale Animal Cell Culture. Cytotechnology 2006; 50, 9–33., [9]Isailovic B, Kradolfer M, Rees B. Fluid Dynamics of a Single-Use Stirred Tank Bioreactor for Mammalian Cell Culture. BioProcess Int. 2015; 13(8), 60–66..
Shear can often be perceived as being a potential cause of damage to the viral vectors once produced. Indeed, once the cells are transfected (or induced in the case of stable cell lines), they start expressing the viral genes and produce and package the vector [4]Sanderson T, Erlandson T, Hazi N et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell Gene Ther. Ins. 2021; 7(9), 1025–1033. In some cases, the vector remains mostly intracellular (AAV2, AAV5 for example), but it can also be completely or partially excreted by the cells into the cell culture media, either through exocytosis or cell lysis caused by the vector production cycle.
Viruses are smaller than cells, and the size difference can have an impact on how these cells or viruses are subject to turbulence in the bioreactor. Lentiviruses are traditionally the most sensitive of the viruses used for gene therapy applications, due to their enveloped nature. They are known to be sensitive to not only shear, but also pH, temperature, salt, and foam generation [10]Perry C, Rayat ACME. Lentiviral Vector Bioprocessing. Viruses 2021; 13(2): 268. .
Through the data illustrated above and a numerous amounts of case studies comparing the Allegro STR bioreactor to other types of STRs, it has been shown there is no damage to AAV integrity [4]Sanderson T, Erlandson T, Hazi N et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell Gene Ther. Ins. 2021; 7(9), 1025–1033, [11]Collignon M-L, Mainwaring D, Pedregal A et al. Targeting Cost-effective Large-scale Manufacturing for rAAV Vectors by Transient Transfection using Pall’s Allegro STR Bioreactor. Poster published at ESGCT 2021., [12]Mainwaring D, Joshi A, Coronel J, Niehus C. Transfer and scale-up from 10L BioBLU® to Allegro™ STR 50 and STR200 Bioreactors. Cell Gene Ther. Ins. 2021; 7(9), 1347–1362..
In a recent Pall study performed with a customer, LV have also been cultured successfully in the Allegro STR bioreactor at 50 L scale, without any specific damage to vector integrity, suggesting the system to be gentle enough to successfully produce these very delicate vectors at any scale. Process performance in the Allegro STR 50 bioreactor was compared to a validated 5 L scale down model. Through replicate runs, metabolite profiles and product physical titer and quality (functional titer and impurities) were reproducible between the two scales [13]Heshmatifar S, Collignon M-L; Kingwood M et al. Evaluation of Pall’s Allegro™ STR Bioreactor Production For Use in Cultivation of Mammalian Suspension Cells and Lentiviral Vector Production. Poster published at ESACT 2022..
Sparging
A constant and adequate supply of oxygen is crucial in cell culture. Allegro STR bioreactor spargers have been designed for optimal gas bubbles generation and distribution allowing good oxygen mass transfer (kLa) with reduced foaming. The most common efficient method for oxygen transfer and carbon dioxide (CO2) stripping across all bioreactor scales is sparging through a ring sparger which was designed with holes of suitable size and number to achieve high flow rates (0.2 vvm) without excessive linear velocities. The system produces relatively large bubbles, which are less likely to damage cells than are small bubbles [7]Nienow AW. Reactor Engineering in Large Scale Animal Cell Culture. Cytotechnology 2006; 50, 9–33. while maintaining high oxygen transfer through adequate specific power and sparge rate. Pall Corporation document reference USD3381 outlines scalable gas transfer coefficients (kLa) and scalable CO2 stripping rates for all bioreactors scales [14]USD3381 – Characterization and Engineering Performance of the Allegro™ STR Single-Use Stirred Tank Bioreactor Family Range. Pall Corporation: East Hills, NY, 2020.
Overall, the Allegro STR spargers have been designed and aligned with the ‘elephant ear’ impeller for optimal gas bubbles generation and distribution allowing good oxygen mass transfer and carbon dioxide strip rates across all STR bioreactor scales.
Conclusions
Pall’s Allegro STR range of single use bioreactors are designed for biotechnology manufacturing. The Allegro STR bioreactors are tested and proven bioreactors in mAb manufacturing [15]USD3384 – Scalability Between the Allegro™ STR 50 and STR 500 Bioreactors in a CHO-S Fed-Batch Process. Pall Corporation: East Hills, NY, 2020. With Pall’s excellent customer support and bioprocess expertise the effective and successful transfer of any gene therapy processes into Pall’s Allegro STR bioreactors is assured. The ability to effectively scale enables speed to clinic in the gene therapy space.
Attention to system design for excellent scalable manufacturing, and usability makes Pall’s range of Allegro STR bioreactors a good choice for viral vector production. Successful testing and adoption by several companies have shown its effectiveness in the gene therapy space.
Collignon et al. transferred an r-AAV process from a competitor 50 L SUB to Pall’s Allegro STR 50 bioreactor, with the objective to scale up to 1000 L. The user friendliness of the system and software, with close support from Pall scientific teams, was noted along with a favorable increase in production yields obtained through a change in dissolved oxygen strategy, thus reducing the overall gas consumption and foam formation [11]Collignon M-L, Mainwaring D, Pedregal A et al. Targeting Cost-effective Large-scale Manufacturing for rAAV Vectors by Transient Transfection using Pall’s Allegro STR Bioreactor. Poster published at ESGCT 2021..
In another study published by Mainwaring et al., a stable AAV producer cell line was successfully transferred from bench scale BioBLU® 10c to the 50 L Allegro STR bioreactor, and further scaled up to the Allegro STR 200 bioreactor. They also demonstrated good capacity, yield, and scalability for the initial unit operations of the downstream process. As a result, processes developed with other manufacturers’ bioreactor can readily be transferred to Allegro STR bioreactors based off known scaling process parameters [12]Mainwaring D, Joshi A, Coronel J, Niehus C. Transfer and scale-up from 10L BioBLU® to Allegro™ STR 50 and STR200 Bioreactors. Cell Gene Ther. Ins. 2021; 7(9), 1347–1362..
As part of the Covid vaccine consortium in 2020, Pall supported the rapid development and scale up of the ChAdOx1 vaccine, an adenovirus-based vaccine. The process was scaled up to the Pall Allegro STR 50 and Allegro STR 200 bioreactors in record time. Pall Allegro STR bioreactors up to 2000 L are consequently being used at various manufacturing sites to successfully produce the vaccine [16]Joe CCD, Jiang J, Linke T et al. Manufacturing a chimpanzee adenovirus-vectored SARA-CoV-2 vaccine to meet global needs. Biotechnol. Bioeng. 2021;1–11..
Ultimately, these case studies show that the Allegro STR bioreactor portfolio leverages decades of process engineering expertise to support successful cell culture in the gene therapy space. The availability of cost-effective gene therapies is critical for wider success of these novel medicines. Platform processes can contribute to that, especially where fully disposable or hybrid manufacturing is adopted.
The Allegro STR’s proven delivery of consistent and scalable cell culture performance can easily be part of the solution.
References
1. Sayed N, Allawadhi P, Khurana A, Singh V et al. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022; 294(2022), 120375. Crossref
2. Gene Therapy Market Size, Growth, Trends, Report 2021–2030. Crossref
3. Nienow AW, Isailovic B, Barrett TA et al. Design and Performance of Single-Use, Stirred-Tank Bioreactors. BioProcess Int. 2016; 14(10), 12–21. Crossref
4. Sanderson T, Erlandson T, Hazi N et al. Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector. Cell Gene Ther. Ins. 2021; 7(9), 1025–1033 Crossref
5. Chahal PS, Schulze E, Tran R, Montes J, Kamen AA. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods 2014; 196: 163–73. Crossref
6. Trivedi PD, Yu C, Chaudhuri P et al. Comparison of highly pure rAAV9 vector stocks produced in suspension by PEI transfection or HSV infection reveals striking quantitative and qualitative differences. Mol. Ther. Methods Clin. Dev. 2021; 24: 154–170. Crossref
7. Nienow AW. Reactor Engineering in Large Scale Animal Cell Culture. Cytotechnology 2006; 50, 9–33. Crossref
8. USD 2980. Application Note: Characterization and Engineering Performance of the Allegro™ STR 200 Single-Use Stirred Tank Bioreactor System. Pall Corporation: East Hills, NY, 2014 Crossref
9. Isailovic B, Kradolfer M, Rees B. Fluid Dynamics of a Single-Use Stirred Tank Bioreactor for Mammalian Cell Culture. BioProcess Int. 2015; 13(8), 60–66. Crossref
10. Perry C, Rayat ACME. Lentiviral Vector Bioprocessing. Viruses 2021; 13(2): 268. Crossref
11. Collignon M-L, Mainwaring D, Pedregal A et al. Targeting Cost-effective Large-scale Manufacturing for rAAV Vectors by Transient Transfection using Pall’s Allegro STR Bioreactor. Poster published at ESGCT 2021. Crossref
12. Mainwaring D, Joshi A, Coronel J, Niehus C. Transfer and scale-up from 10L BioBLU® to Allegro™ STR 50 and STR200 Bioreactors. Cell Gene Ther. Ins. 2021; 7(9), 1347–1362. Crossref
13. Heshmatifar S, Collignon M-L; Kingwood M et al. Evaluation of Pall’s Allegro™ STR Bioreactor Production For Use in Cultivation of Mammalian Suspension Cells and Lentiviral Vector Production. Poster published at ESACT 2022. Crossref
14. USD3381 – Characterization and Engineering Performance of the Allegro™ STR Single-Use Stirred Tank Bioreactor Family Range. Pall Corporation: East Hills, NY, 2020 Crossref
15. USD3384 – Scalability Between the Allegro™ STR 50 and STR 500 Bioreactors in a CHO-S Fed-Batch Process. Pall Corporation: East Hills, NY, 2020 Crossref
16. Joe CCD, Jiang J, Linke T et al. Manufacturing a chimpanzee adenovirus-vectored SARA-CoV-2 vaccine to meet global needs. Biotechnol. Bioeng. 2021;1–11. Crossref
Affiliations
Emmanuelle Cameau
Pall Corporation
Ernest Asilonu
Pall Corporation
Sheriff Bah
Pall Corporation
Pauline Nicholson
Pall Corporation
Timothy Barrett
Pall Corporation
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors disclose that Pall Corporation owns patents relevant to the Bio-tech Industry.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Pall Corporation, Pall Europe Limited, Pall France SAS. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Jun 16 2022; Revised manuscript received: Jul 27 2022; Publication date: Sep 1 2022.

