Methylcellulose as a scaffold in the culture of liver-organoids for the potential of treating acute liver failure
Cell Gene Therapy Insights 2018; 4(11), 1087-1104.
10.18609/cgti.2018.111
Much progress has been made in understanding the development of human organs through advanced cellular and molecular techniques. Acute liver failure (ALF) in children is a life-threatening condition that relies on liver or hepatocyte transplantation. The translation of novel regenerative medicine strategies for the treatment of ALF is however somewhat limited. Here, we show that in vitro liver-organoids derived from human umbilical cord derived mesenchymal stem cells and human cadaveric donor-derived hepatocytes, cultured in a clinically appropriate manner, exhibit liver function. We obtained organoids that varied in size and morphology which produced albumin, and detoxified ammonium chloride into urea. Immunohistochemistry of these organoids revealed hepatocyte specific, non-parenchymal markers and histological organisation. Our in vitro findings indicate that these organoids may be a useful bridge in ALF while awaiting liver recovery or transplant. The organoid culture system we have established here is also well suited to drug screening and disease modeling.
Submitted for Peer Review: 8 Aug 2018 Published: 18 Dec 2018
Introduction
There have been significant conceptual and practical advances with regards to regenerative medicine for the treatment of disease. This has ranged from the classic use of embryonic stem cells, their culture and ability to differentiate into practically any cells of the body, to reprogramming of fully differentiated cells into an embryonic state followed by differentiation to a chosen cell type, which is now mainstream re-search [1]. These now significantly impact not only on treat-ing disease but the potential for modelling disease in culture dishes that was previously not possible. The development of liver has been of prominent interest, as liver disease is the fifth biggest cause of deaths in the UK.
Acute liver failure (ALF) in children is a life-threatening condition. The prognosis of ALF is generally poor and medical intervention relies on a liver [2,3] or hepatocyte [4,5] transplantation if the native liver is unable to recover. The major limitation of the technique today is the availability of donor organs. Many patients who might benefit from a transplant die before a suitable organ can be found. Whilst much progress has been made in the study and understanding of the biology of liver [6], there have been limited clinical advancements in the treatment of ALF utilizing novel regenerative techniques, such as the use of embryonic stem cell techniques to induced pluripotent stem cell-derived hepatocytes. Human bio-compatible scaffolds to grow liver type/functional cells is ever increasing. The main challenges in translating advances in basic science of cell therapy into the clinic has been determining the best route of delivery, the rapid elimination of transplanted cells by the recipient, poor engraftment and proliferation of transplanted cells within the liver [7].
Stem cell-based technologies have been extensively researched in vitro and in vi-vo. Mesenchymal stem/stromal cells (MSCs) are a population of immature cells that can give rise to more mature cell types such as adipogenic, osteogenic and chondrogenic cells. These cells can be easily cultured from adult bone marrow and full-term umbilical cord (blood or the Wharton’s jelly). MSCs have also been isolated from muscle connective tissue, adipose tissue and in some circumstances periph-eral blood. Culture protocols usually include direct plastic adherence or enrichment by CD marker selec-tion. The well-established characteristics of MSCs, have been the basis of the extensive use in clinical applications. MSCs have been used in the treatment of liver disease with varying results [8].
Tissue engineering strategies for regenerative medicine are yet another avenue of great advancement. Novel scaffolds-engineered [9], naturally occurring [10] or synthetic material [11] are under extensive scrutiny. There is an unmet need to use novel organoid-based strategies that is suitable for transplantation in ALF until the patient receives a liver transplant or the native liver recovers. As such, we proposed to exploit novel organoid-strategies as a means for clinical application. Here we hypothesized that using methylcellulose as a scaffold to obtain liver-organoids maybe a useful approach towards clinical use.
Results
Establishment of organoids in matrigel
Our initial strategy to develop liver organoids, stems from the seminal research of Takebe and colleagues [12]. Whilst Takebe et al. used human induced pluripotent stem cell (hiPSC) derived hepatocytes, MSCs and human umbilical vein endothelial cells (HUVECS) cultured in matrigel with a host of factors to obtain organoids, we used adult hepatocytes from cadaveric donors and MSCs cultured in serum free matrigel. Organoids were analyzed after 14 days in culture (Figure S1A). Confocal imaging of these organoids revealed strong expression of hepatocyte markers (Figure S1B). Our rationale for the ratio of cells to be cultured was based on our previous experience of a 2-D co-culture system of hepatocytes and MSCs, which resulted in improved hepatocyte function [13]. We tested four different ratios of hepatocytes cultured with MSCs. Albumin production and ability to metabolise ammonium chloride to urea were used as functional readouts of the resulting organoids after two weeks in culture. Hepatocytes and MSC cultured in a two to one ratio respectively, gave rise to the highest albumin production (Figure S2A). Interestingly, the urea production was the highest when using a one to one ratio (Figure S2B). When using a two to one ratio of cultured cells, these organoids produced albumin over a period of 2 weeks, peaking from day 10 onwards (Figure S3A). These organoids were also able to metabolise ammonium chloride to produce urea from day 4 peaking at day 14 as well (Figure S3B).
Establishing organoids using methylcellulose as a scaffold
Matrigel whilst proving to be an excellent scaffold for studying and understanding basic biology of organ development, its clinical application is somewhat restricted as it is a gelatinous substance derived from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells. Therefore, we hypothesized that substituting matrigel with methylcellulose as a scaffold for the growth of organoids might be a useful approach to using organoids for future clinical application (Figure S4). Having studied the composition of growth factors present in matrigel (Corning), we tested the effects of transforming growth factor-ß?(TGF-ß), hepatocyte growth factor (HGF) and epidermal growth factor (EGF); individually and in combination, in methylcellulose for the ability to produce organoids. A two-part hepatocyte to one-part MSC ratio were maintained and cultured in the presence of methylcellulose and growth factors in non-tissue culture 6-well plates. When we cultured MSC and hepatocytes with a single cytokine only, no organoids formed (Figure 1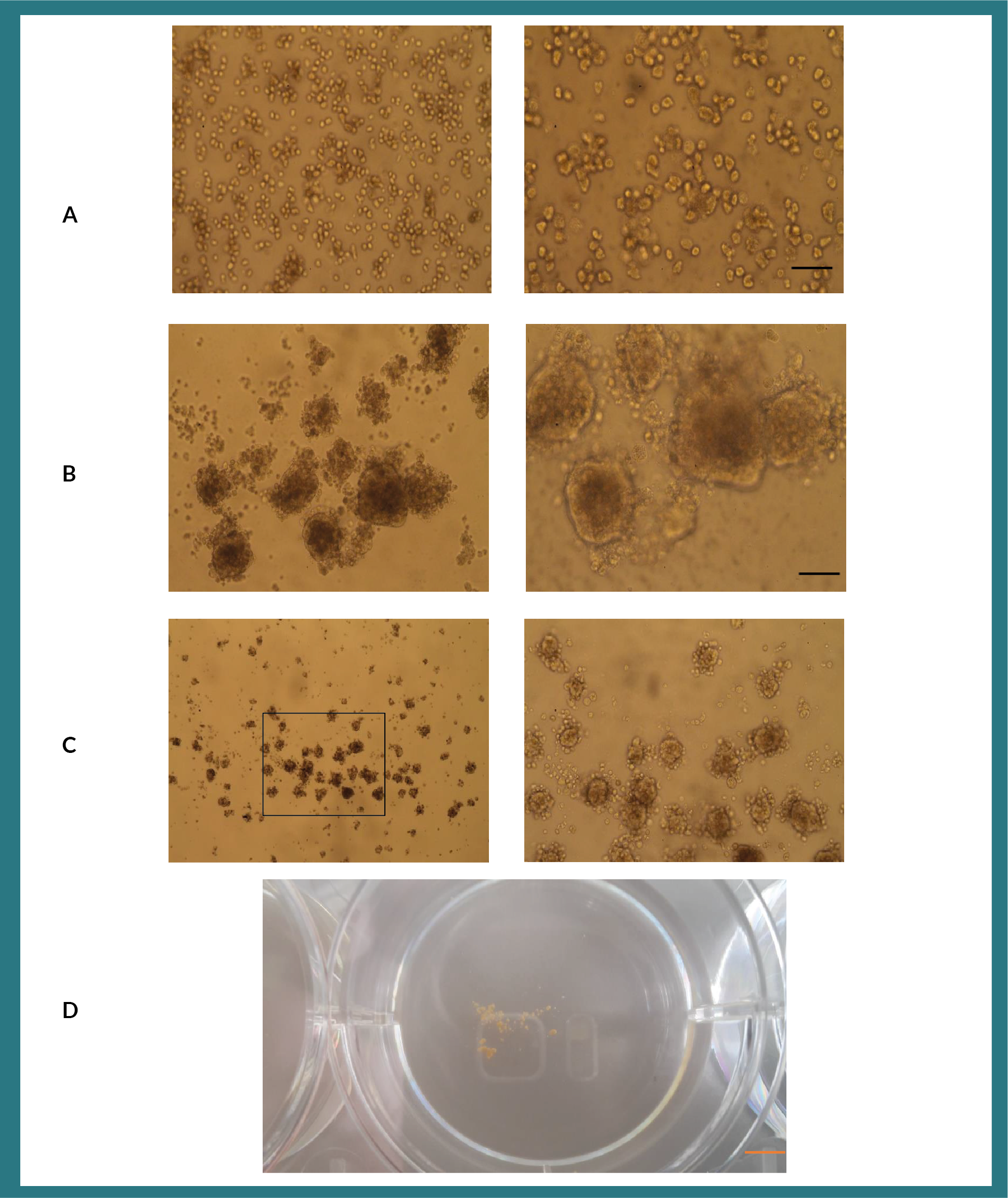
Liver function activities of organoids cultured in methylcellulose
We next tested the ability of organoids to produce albumin and detoxify ammonium chloride, in optimally cultured in the conditions. We compared these conditions, to that of organoids obtained using matrigel as a reference point. Albumin production was highest from organoids derived from cultures containing TGF-ß and EGF (Figure 2A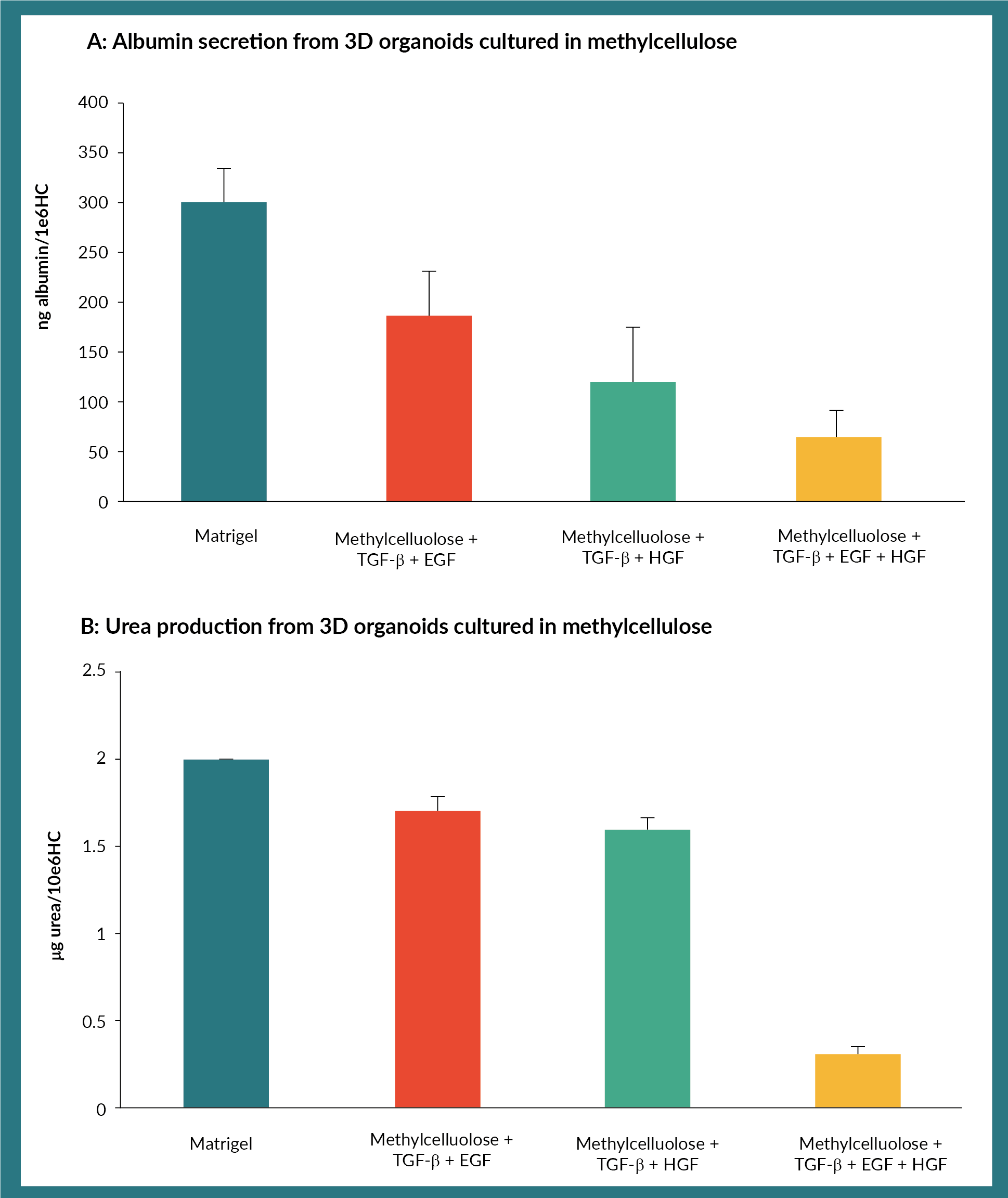
Histological & biochemical properties of organoids derived from methylcellulose
Having established the functionality of these organoids, we then analysed the histology of these optimally cultured organoids. These organoids expressed hepatocyte specific marker, OCH1E5 and; biliary marker, cytokeratin 7 (Figure 3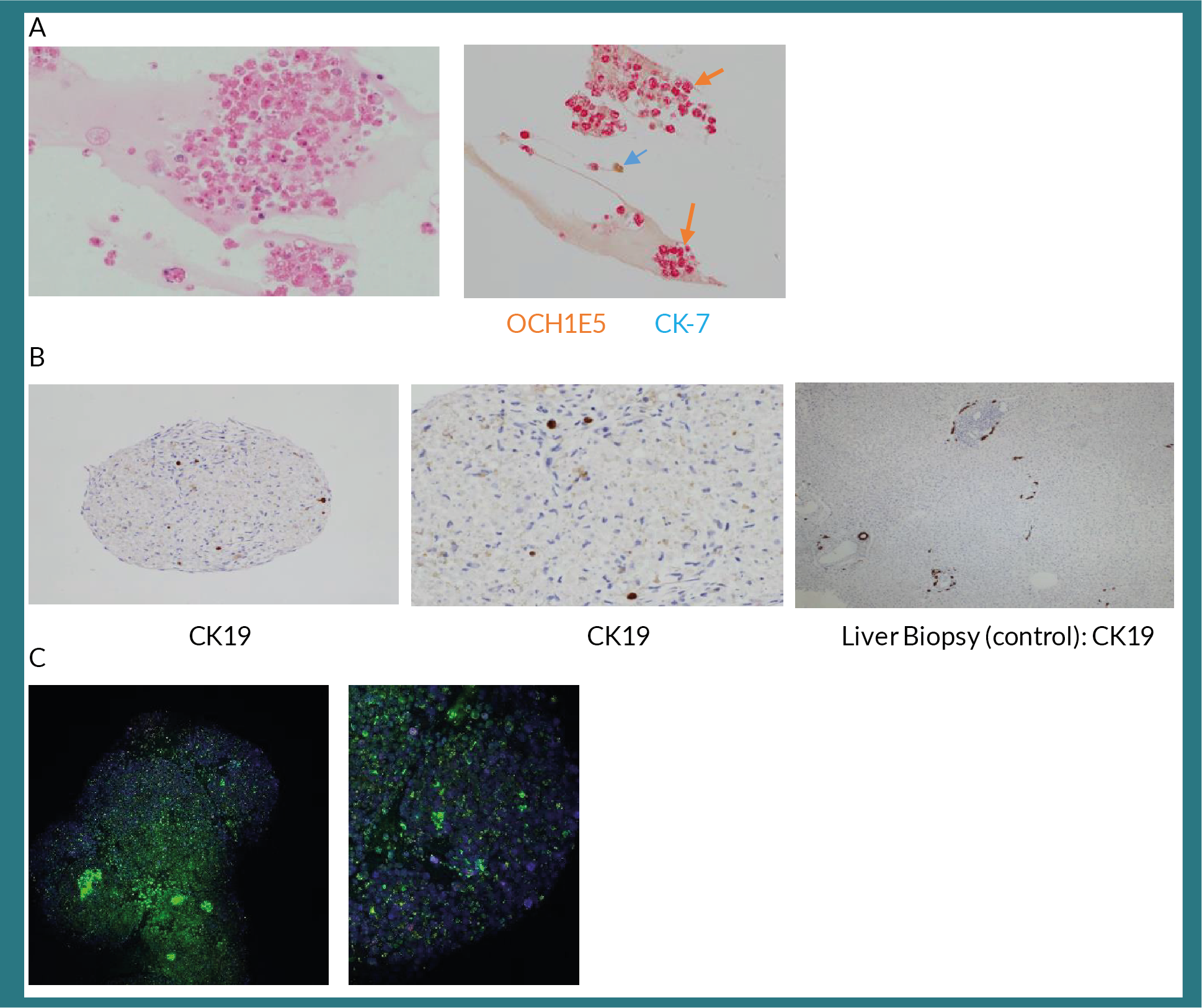
Finally, we assessed the ability of organoids to conjugate bilirubin. Albeit relatively lower levels com-pared to the HepG-2 cell line, these organoids could conjugate bilirubin (Figure 4A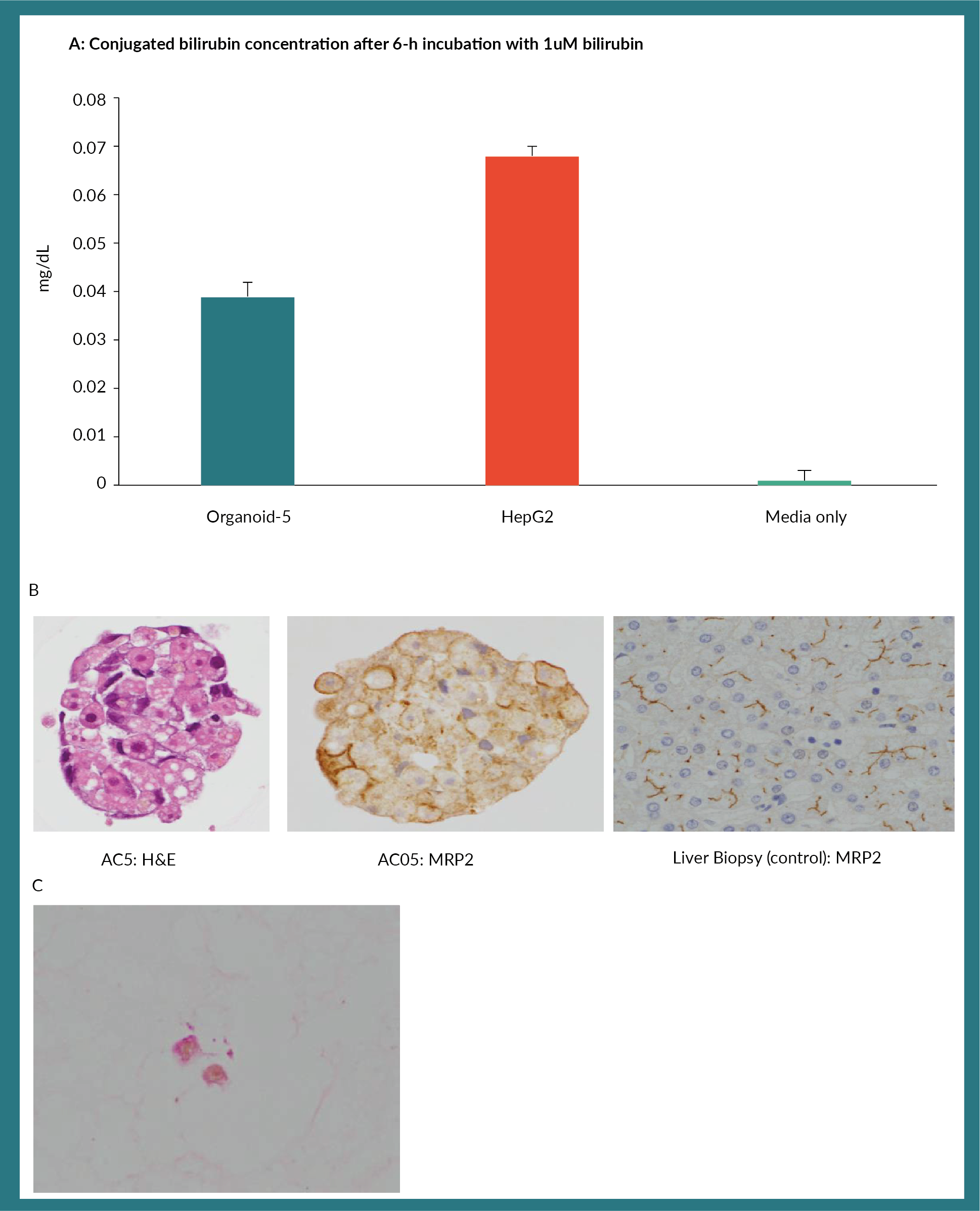
Discussion
In this study, we show that methylcellulose, an inert semi-solid media, could be utilized as a scaffold to establish liver-organoids that resemble liver structure and function. The results we obtained here are comparable to that of using matrigel as a scaffold. There was also a good correlation of the morphology of organoids obtained to functional activity. Albeit the lowered functional activities (albumin and urea production) using methylcellulose as opposed to matrigel, the suitability of using methylcellulose towards clinical grade expansion of organoids is highly compelling. Further optimization and scaling up of the process is underway. Methylcellulose is a synthetic chemical product derived from cellulose. Cellulose is heated with sodium hydroxide and the resulting substitution of hydroxyl residues on cellulose are re-placed with methoxide [14]. Methylcellulose is used in a wide array of applications in industry and in the clinic. It is a well-known agent for the treatment of constipation [15] and its derivatives can be used as artificial tears [16,17] or saliva. In the laboratory, methylcellulose has long been used as a scaffold for the development of colony forming cells from haematopoietic stem/progenitor cells and is a good support for studying stem cell proliferation and differentia-tion [18–20]. As such, it should be relatively easy to implement a clinical grade strategy using methylcellulose as a scaffold to obtain liver-organoids.
A further advantage to using methylcellulose as a scaffold is the flexibility to assess the effects of different growth factors, tailor-made chemicals or synthetic combinations on organoid formation. We found that the use of EGF and TGF-ßtogether were crucial in establishing organoids. EGF is a member of the tyrosine kinase receptor family [21] and is important in the upregulation of MAP kinase signalling pathway leading to DNA synthesis and cell proliferation [22]. TGF-ß on the other hand is a growth factor known to induce apoptosis in lymphocytes and hepatocytes in mice [23]. It is also thought to stop proliferation and induce differentiation in stem cells [24]. It is intriguing that the synergistic effect of EGF and TGF-ß in our experiments was necessary for the formation of organoids, to which the exact mechanism of organoid development remains to be determined. The simplicity of this system means that it can also be scaled up for drug screening and cell signalling experiments as well.
We and others have previously shown that hepatocytes isolated from a donor liver, can be used to treat children with ALF effectively [25–27]. Further improvements to hepatocyte transplantation has been the method of encapsulating hepatocytes with alginate beads [28,29]. This encapsulating method eliminates the need for immunosuppressing drugs that are routinely used in liver or non-encapsulated hepatocyte transplantation. Such alginate en-capsulated human hepatocyte transplants in humans although not necessarily curative, have shown to be useful as a bridge to native liver recovery or until a suitable donor liver is found [Anil Dhawan, Personal Communication/Unpublished Data].
Recently, bio-fabrication techniques have been developed using 3D plotting with methylcellulose and alginate. MSCs cultured in these 3D scaffolds retained viability and differentiation properties [30,31]. Taken together, it is highly conceivable to be able to establish organoids in methylcellulose, as done here and then encapsulating the resulting organoids in alginate-methylcellulose 3D scaffolds [32]. Such a strategy should be highly applicable in the treatment of ALF.
Methods
Hepatocyte & MSC isolation
Primary hepatocyte isolation and research activities were ethically approved via the National Research Ethics Service (King’s College Hospital local research ethics committee; LREC 01-016). Organs were donated through the National Health Service Blood and Transplant (NHSBT) and offered for hepato-cyte transplantation and/or research following decline for solid organ transplantation. Hepatocytes were derived from donor as previously described [33]. All hepatocyte isolation procedures were performed to Good Manufacturing Practises (GMP) standards (Cell Therapy Unit, King’s College Hospital) and governed by the Human Tissue Authority for the storage, clinical and research use of these cells. Briefly, surgically obtained split liver lobes were weighed and cannulated [26]. This was followed a by an in-house modified collagenase perfusion step, followed by a perfusion to inactivate the collagenase. Perfused liver lobes were then macerated using scissors and forceps and filtered through gauze until clumps had reduced. Hepatocytes were obtained by centrifugation at 50g, where the hepatocyte fraction was collected from the cell pellet. Cell number and viability was determined by trypan blue exclusion. Cells were frozen in cryovials (re-search use) and cryobags (clinical use), using a controlled rate freezer and stored at -180°C vapour phase nitrogen freezers.
MSCs were isolated from Wharton’s jelly (WJ) of umbilical cords obtained following caesarean sec-tion deliveries at King’s College Hospital. These umbilical cords were procured via the Anthony Nolan Trust, according to King’s College Hospital Institutional guidelines. MSCs were isolated at research laboratory grade standards. Briefly, cords were collected in PBS containing 40µg/ml of gentamicin [34]. Cords were cut into sections to expose the WJ, which was minced into fine pieces (1–3 mm2). The explants were placed in sterile petri dishes and immersed with MSC culture medium, consisting of MEM ? (Life Technologies), 5% Stemulate (Cook Regentec), 40µg/ml of gentamicin and allowed to adhere for up to 5 minutes. Cord samples were then incubated at 37°C, 5% CO2 in atmosphere and half medium replenished every 3–6 days. Gentamicin was used in the MSC medium for the first 1–2 weeks of culture and then replaced by penicillin/streptomycin (100 units/ml and 100 µg/ml, respectively). Once cell cultures were established, they were expanded, quality controlled and cryopreserved. Cells were positive for MSC markers (i.e., CD73, CD90 and CD105; expression higher than 75%) and negative for hematopoietic markers (i.e., CD14, CD34 and CD45; expression lower than 2%) by flow cytometry.
Organoid formation
The initial establishment of liver organoids was adapted from Takebe et al. Briefly, hepato-cytes and MSC were thawed and cultured in round bottom non-tissue culture treated 96 well plates. The culture media consisted of DMEM/F12 high glucose (Gibco) as the basal media supplemented with N2 (Gibco) and B27 (Gibco) supplements, Penicillin and Streptomycin and L-glutamine. Matrigel (Corning) was added to a final concentration of 1%. The initial ratio of hepatocytes to MSC was 1:1 and 105 cells in 200 µl total volume used. Plates were incubated at 37°C, 5% CO2 in atmosphere. Different cell ratios were investigated. Plates were cultured for 2 weeks and monitored daily for the growth of organoids.
Having established the system in Matrigel, methylcellulose (BioTechne) was substituted as a scaffold. We also scaled up the process into non-tissue culture treated six well plates, omitting the use of Penicillin and Streptomycin. Methylcellulose was added to a similar culture media as above to a final concentration of 1.2%. 10ng/ml final concentration of TGF-ß, HGF and EGF (all from BioTechne) was used in cultures, either individually and/or in combination. Plates were incubated at 37°C in 5% CO2 in atmosphere. Plates were cultured for 2 weeks and monitored daily for the growth of organoids.
Albumin assay
Albumin secretion from organoids were measured by a standard ELISA protocol as previously described [13]. Briefly, organoids were transferred to fresh plates and incubated with media (less the scaffold) for 8 to 18 hours, and media was then removed and stored at -20°C until the assay.
Urea assay
Urea assay was performed according to previously established methods [13]. Again, organoids were transferred to new plates and incubated with media (less scaffold) in the presence of 4mM Ammonium Chloride. Urea production in the media was meas-ured 6 hours later using QuantiChrome Urea Assay Kit (BioAssay Systems, Cambridge, UK).
Bile conjugation assay
Total and conjugated bile was determined by using a bilirubin assay kit from Sigma. One µM unconjugated bilirubin was used to spike organoids. This assay is based on the Jendrassik-Grof method which utilizes the reaction of bilirubin with diazotized sulfonic acid resulting in a colorimetric product measured at 530 nm, proportionate to the bilirubin present in the sample.
Histology
Developing and established organoids were fixed in 4% paraformaldehyde. Fixed organoids were embedded in 2.5% agarose. The set agarose-organoid material was then routinely processed. Five-micron thin paraffin embedded sections were placed on poly-lysine coated slides. Standard immunohistochemistry protocols were employed. Details of antibodies used in Table S1. All immune-stained sections were counter stained with Haematoxylin and permanently mounted.
Confocal microscopy was performed on live organoids mildly permeabilised with 0.01% triton-x in PBS. Organoids were then stained with respective antibodies (Table S1), washed twice in PBS and mounted onto glass slides. Confocal microscopy was performed on a Leica SP5 microscope.
Acknowledgements
We would like to thank Ms Shirin E Khorsandi for reading the manuscript. The authors would also like to thank Mr Simon Walker (MSC and hepatocyte isolation), Dr Charlotte Lee and Dr Raquel Fernandez-Dacosta for help with hepatocyte isolations. Our gratitude also goes to all the families of the liver donors for consenting to the use of tissue for research and the liver transplant coordinators at King’s College Hospital London for their tireless work. This work was funded by the Medical Research Council, UK under the UK Regenerative Medicine Pro-gramme-II; Stem Cells and the Niche. Ms Simona Asan, a research student involved in the project passed away shortly before drafting the paper and this work is dedicated in her memory.
author contributions
Anil Chandrashekran, Anil Dhawan conceived and designed experiments; Anil Chandrashekran, Tharindu Premachandra, Chris Starling performed experiments; Anil Chandrashekran, Maesha Deheragoda, Anil Dhawan analyzed and interpreted data; Sharon Lehec, Ragai R Mitry, Celine Filippi, Valeria Iansante, Anil Chan-drashekran for GMP hepatocyte isolation; Valeria Iansante responsible for MSC isolation; SB, Emer Fitzpatrick obtained and provided clinical samples; Ragai R Mitry, Celine Filippi, David Hay provided useful discussion, in-sight and support. Anil Chandrashekran, Anil Dhawan wrote the paper.
financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Daley GQ. Stem cells and the evolving notion of cellular identity. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015; 370(1680): 20140376.
2. Rela M, Vougas V, Muiesan P et al. Split liver transplantation: King’s College Hospital experi-ence. Ann. Surg. 1998; 227(2): 282–8. CrossRef
3. Bonatti H, Muiesan P, Connolly S et al. Liver transplantation for acute liver failure in children under 1 year of age. Transplant. Proc. 1997; 29(1-2): 434–5. CrossRef
4. Strom SC, Fisher RA, Thompson MT et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation 1997; 63(4): 559–69. CrossRef
5. Bilir BM, Guinette D, Karrer F et al. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000; 6(1): 32–40. CrossRef
6. Best J, Manka P, Syn WK, Dolle L, van Grunsven LA, Canbay A. Role of liver progenitors in liver regenera-tion. Hepatobiliary Surg. Nutr. 2015; 4(1): 48–58.
7. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2010; 7(5): 288–98. CrossRef
8. Iansante V, Chandrashekran A, Dhawan A. Cell-based liver therapies: past, present and future. Philos. Trans. R Soc. Lond, B Biol. Sci. 2018; 373(1750).
9. Ghaedi M, Soleimani M, Shabani I, Duan Y, Lotfi AS. Hepatic differentiation from human mesenchymal stem cells on a novel nanofiber scaffold. Cell Mol. Biol. Lett. 2012; 17(1): 89–106. CrossRef
10. Maghsoudlou P, Georgiades F, Smith H et al. Optimization of Liver Decellularization Main-tains Extracellular Matrix Micro-Architecture and Composition Predisposing to Effective Cell Seeding. PLoS One 2016; 11(5): e0155324. CrossRef
11. Kelly CN, Miller AT, Hollister SJ, Guldberg RE, Gall K. Design and Structure-Function Characterization of 3D Printed Synthetic Porous Biomaterials for Tissue Engineering. Adv. Healthc. Mater. 2017.
12. Takebe T, Sekine K, Enomura M et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013; 499(7459): 481–4. CrossRef
13. Fitzpatrick E, Wu Y, Dhadda P et al. Coculture with mesenchymal stem cells results in im-proved viability and function of human hepatocytes. Cell Transplant. 2015; 24(1): 73–83. CrossRef
14. Nasatto PL, Pignon F, Silveira JLM, Duarte MER, Noseda MD, Rinaudo M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers 2015; 7(5): 777–803. CrossRef
15. Snape WJ Jr. The effect of methylcellulose on symptoms of constipation. Clin. Ther. 1989; 11(5): 572–9.
16. Yusufu M, Liu X, Zheng T, Fan F, Xu J, Luo Y. Hydroxypropyl methylcellulose 2% for dry eye prevention during phacoemulsification in senile and diabetic patients. Int. Ophthalmol. 2017.
17. Safarzadeh M, Azizzadeh P, Akbarshahi P. Comparison of the clinical efficacy of preserved and preservative-free hydroxypropyl methylcellulose-dextran-containing eyedrops. J. Optom. 2017; 10(4): 258–64. CrossRef
18. Chandrashekran A, Gordon MY, Casimir C. Targeted retroviral transduction of c-kit+ hematopoietic cells using novel ligand display technology. Blood 2004; 104(9): 2697–703. CrossRef
19. Lewis JL, Chinswangwatanakul W, Zheng B et al. The influence of INK4 proteins on growth and self-renewal kinetics of hematopoietic progenitor cells. Blood 2001; 97(9): 2604–10. CrossRef
20. Eaves CJ, Sutherland HJ, Udomsakdi C et al. The human hematopoietic stem cell in vitro and in vivo. Blood Cells 1992; 18(2): 301–7.
21. Herbst RS. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004; 59(2 Suppl.): 21–6. CrossRef
22. Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005; 1: 2005 0010.
23. Kulkarni AB, Huh CG, Becker D et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA 1993; 90(2): 770–4. CrossRef
24. Massague J, Xi Q. TGF-beta control of stem cell differentiation genes. FEBS Lett. 2012; 586(14): 1953–8. CrossRef
25. Bartlett A, Vara R, Muiesan P et al. A single center experience of donation after cardiac death liver transplantation in pediatric recipients. Pediatr. Transplant. 2010; 14(3): 388–92. CrossRef
26. Mitry RR, Dhawan A, Hughes RD et al. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation 2004; 77(10): 1614–6. CrossRef
27. Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr. Res. 2017.
28. Jitraruch S, Dhawan A, Hughes RD et al. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS One 2014; 9(12): e113609. CrossRef
29. Mitry RR, Jitraruch S, Iansante V, Dhawan A. Alginate Encapsulation of Human Hepatocytes and As-sessment of Microbeads. Methods Mol. Biol. 2017; 1506: 273–81. CrossRef
30. Li H, Tan YJ, Leong KF, Li L. 3D Bioprinting of Highly Thixotropic Alginate/Methylcellulose Hydrogel with Strong Interface Bonding. ACS Appl. Mater. Interfaces 2017; 9(23): 20086–97. CrossRef
31. Schutz K, Placht AM, Paul B, Bruggemeier S, Gelinsky M, Lode A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: towards biofabrication of tissue engineering constructs with clinically relevant di-mensions. J. Tissue Eng. Regen. Med. 2017; 11(5): 1574–87. CrossRef
32. Payne C, Dolan EB, O’Sullivan J, Cryan SA, Kelly HM. A methylcellulose and collagen based temperature responsive hydrogel promotes encapsulated stem cell viability and proliferation in vitro. Drug Deliv. Transl. Res. 2017; 7(1): 132–46. CrossRef
33. Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: isolation, culture & cryopreservation. Semin. Cell Dev. Biol. 2002; 13(6): 463–7. CrossRef
34. Devito L, Badraiq H, Galleu A et al. Wharton’s jelly mesenchymal stromal/stem cells derived under chemically defined animal product-free low oxygen conditions are rich in MSCA-1+ subpopulation. Regen. Med. 2014; 9(6): 723–32. CrossRef
Affiliations
Anil Chandrashekran1,2*, Ragai R Mitry1, Tharindu Premachan-dra3, Chris Starling3, Sharon Lehec1, Valeria Iansan-te1, Emer Fitzpatrick1, Celine Filippi1, Maesha Deherago-da3, David Hay4 & Anil Dhawan1*
1Paediatric Liver GI and Nutrition Centre and Mowat Labs, King’s College London, 3rd Floor, Cheyne Wing, Denmark Hill, London, SE5 9PJ, UK
2MRC Centre for Regenerative Medicine, University of Edinburgh, 5 Little France Drive, Edinburgh, EH16 4UU, UK
3 Liver Histopathology, Institute of Liver Studies, King’s College Hospital, Denmark Hill, London SE5 9PJ, UK
4Molecular Haematology, School of Cancer & Pharmaceutical Sciences, King’s College London, The Rayne Institute, 123 Coldharbour Lane, London, SE5 9NU, UK
*Authors for correspondence:
anil.chandrashekran@kcl.ac.uk
anil.dhawan@kcl.ac.uk
