Achieve significantly increased adenovirus yield with density gradient ultracentrifugation: a comparative study
Cell & Gene Therapy Insights 2022; 8(8), 1257–1266
DOI: 10.18609/cgti.2022.184
Adenoviral vectors are highly effective tools for gene therapy due to their high gene transduction efficiencies, safety, and tunability. Although numerous technologies exist for the downstream purification of adenovirus, several critical manufacturing challenges still exist, including scalability, low recovery, and the removal of impurities. Two of the most prominent adenovirus purification techniques include density gradient ultracentrifugation (DGUC) and ion exchange chromatography (IEC). Although IEC is well established as a scalable approach to biomolecule purification with short separation times, it suffers from several limitations in the context of adenoviruses, including low binding capacity, serotype dependency, and a trade-off between yield and purity. DGUC, however, is a robust, serotype-independent method that offers improved product recovery by exploiting subtle differences in the buoyant density between full particles and process impurities. In this article, both techniques are directly compared, and significant improvements in product critical quality attributes (CQAs) including more than 200-fold increases in yield and concentration are observed for the DGUC-purified AdV5. In addition, universally applicable approaches to maximize throughput and efficiency when scaling up or down adenovirus production are explored.
Within the field of viral vectors, there are several different therapeutic mechanisms that are exploited. Vectors are used as delivery vehicles in gene therapy, as viral vaccines to deliver genetic material to provide immunity, and as replication-competent viruses in oncolytic virotherapy. Within gene therapy, adeno-associated virus (AAV), adenovirus and lentivirus are all promising vectors for clinical applications.
Nearly half of all gene therapy trials on the Wiley database employ adenovirus. Adenoviruses have the advantage of large size, with a diameter of ~90 nm, providing a large packaging capacity for ~8kb of double-stranded DNA. They also have a high transduction efficiency with a variety of cell types. They are non-integrating vectors, meaning they do not integrate their genetic material into the genome of the patient. Additionally, there is a wide diversity of seven serotype subgroups composed of over 50 different serotypes, enabling high tissue tropism.
With the increasing demand for gene therapies, producing enough viral vectors is a key challenge throughout development. Despite steady innovations in both the upstream and downstream workflows, supply has not kept up with demand. This will likely continue as larger clinical studies are conducted and more prevalent diseases are targeted. Both the number of patients and the required dosing regimens are factors that contribute to the demand for viral vectors.
For example, there are roughly 2000 new cases of spinal muscular atrophy (SMA) per year, and treating all of these patients with Zolgensma translates to a requirement of 42000 L of cell culture. There is also an immense manufacturing burden for the treatment of amyotrophic lateral sclerosis (ALS), with a requirement of nearly 500000 L of cell culture. While scale is a critical component of meeting these demands, process yield should not be overlooked.
Downstream purification of adenovirus
In the workflow of a generic viral vector manufacturing process, a cell bank is first expanded. The rest of the upstream workflow includes infection and subsequent production of viral particles, followed by cell harvest and lysis. The lysate is clarified and the virus particle is purified before formulation and vial fill. Adenovirus is prone to inefficient packaging of DNA cargo, which leads to empty and full particles. Therefore, in downstream purification, enrichment for full particles is an essential objective.
DGUC is a high-resolution purification method that separates particles based on their buoyant density. A defined amount of a density gradient-forming material, such as cesium chloride (CsCl), is added to the tube and spun. After some time, a density gradient forms and the particles of interest migrate to the position where their density is equal to the surrounding media (isopycnic point). In the density gradient, band position directly correlates to particle fullness, meaning denser (fuller) particles will sit lower in a density gradient. DGUC offers high purity separation and extremely high yields in a serotype-agnostic process. Protocols can be directly translated from one serotype to the next, and multiple serotypes can be purified in a single run.
Anion exchange chromatography (AEX) is another popular option for purifying adenovirus. AEX separates capsids based on charge. Charge differences in isoelectric point arise from the presence or absence of the DNA cargo. The AEX resin is functionalized with cations that bind adenovirus. After washing, elution is achieved by flushing the column with a buffer containing high concentrations of counter anions, which displace the virus. AEX is a highly scalable and automatable process.
Case study: quality & yield of AdV5 purified via DGUC versus AEX
In this study,the primary objective was to compare DGUC and AEX for particle recovery and yield, functionality or infectivity, and other critical quality attributes (CQAs). The human adenovirus type 5 (AdV5) purification processes used either AEX or DGUC, as outlined in Figure 1 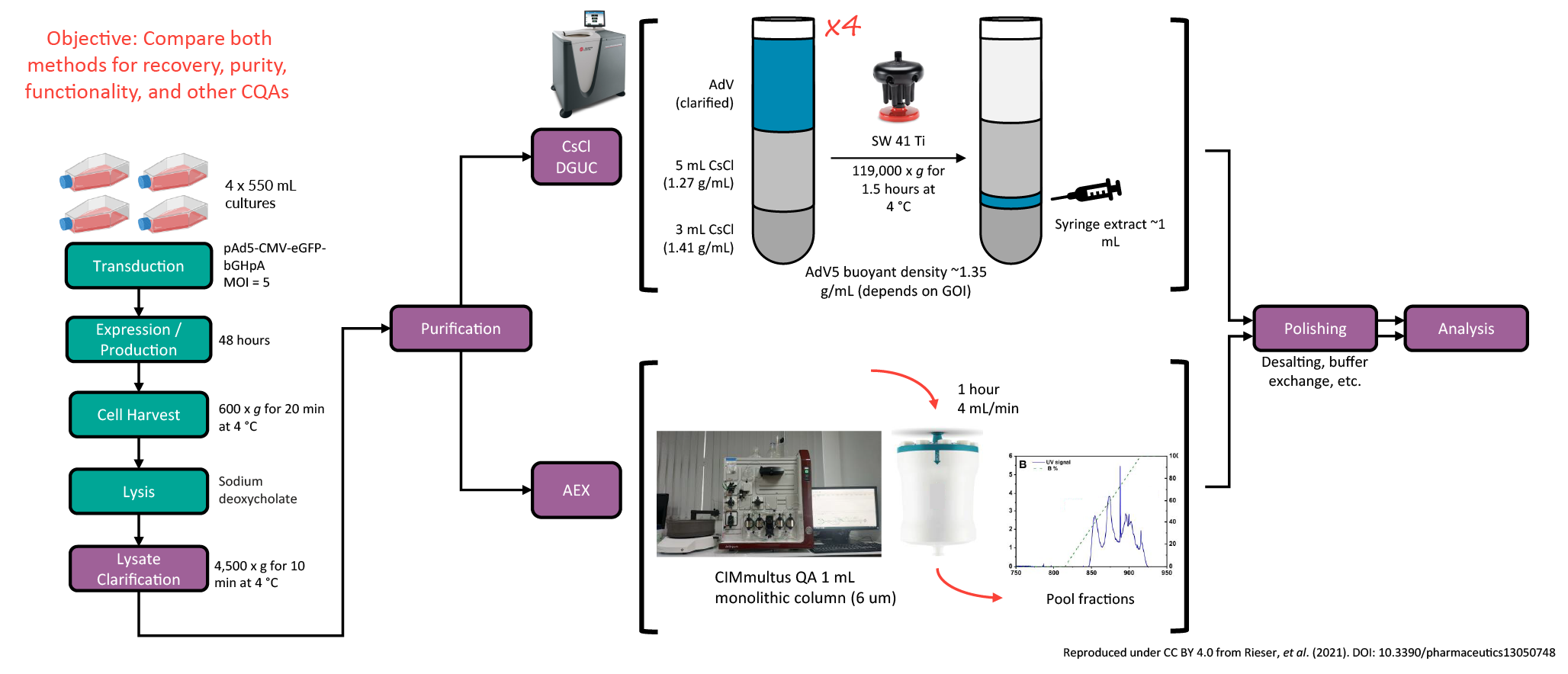 Experimental process for AdV5 purification using DGUC versus AEX. . Yield was quantified using immunohistochemical detection, ddPCR, and OD260 and the results are shown in Figure 2
Experimental process for AdV5 purification using DGUC versus AEX. . Yield was quantified using immunohistochemical detection, ddPCR, and OD260 and the results are shown in Figure 2 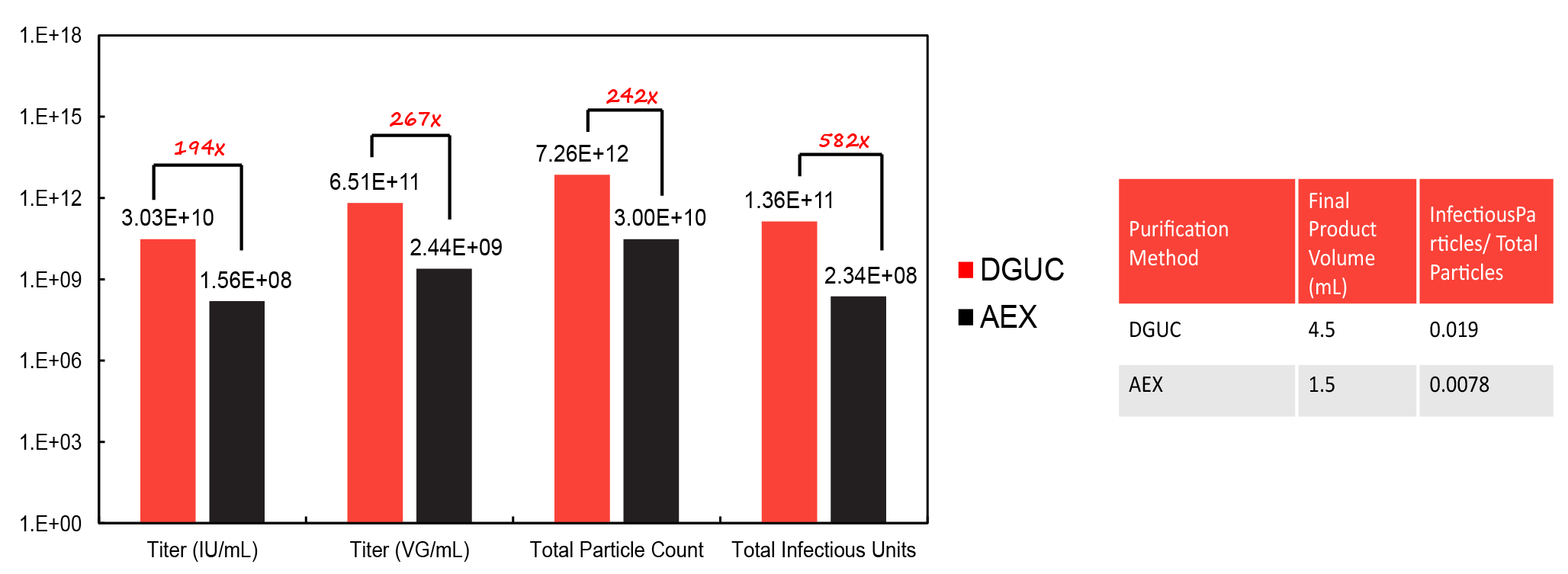 Performance data for titer, total particle count, and total infectious units for AdV5 purified via DGUC versus AEX. .
Performance data for titer, total particle count, and total infectious units for AdV5 purified via DGUC versus AEX. .
This study found that DGUC yields significantly more AdV5 than AEX. DGUC provides a 582-fold increase in the number of infectious particles recovered, as well as a higher ratio of infectious particles to total particles.
DGUC was also shown to give rise to more infective adenovirus particles and improved endotoxin removal. DGUC produced adenovirus particles with ~28% higher functionality and was more efficient at removing endotoxin even at a higher viral concentration.
With both purification schemes, no bacteria or mycoplasma contaminants were detected in either sample. However, endotoxin was detected at 1.1 units per mL in the AEX sample, whilst undetectable in DGUC. Residual host cell proteins were detected in the DGUC sample, but when the sample was diluted to the same adenovirus concentration as the AEX sample, the level was well below the limit of detection.
DGE-AUC characterization
The samples were also analyzed using density gradient equilibrium analytical ultracentrifugation (DGE-AUC). In a manner analogous to preparative DGUC, DGE-AUC employs CsCl to form a gradient and separate particles according to their buoyant density.
Absorbance and interference optics in Beckman Coulter’s AUC instrument, the Optima AUC, allows the monitoring of gradient formation and directly measures the signal at radial positions once equilibrium is achieved. Using a six-sector centerpiece, it is possible to run up to 21 samples simultaneously and achieve equilibrium in 1 h. The chosen run speed affects the slope and the range of the gradient that forms. Higher speeds tend to offer higher sensitivity, while lower speeds favor better resolution.
As shown in Figure 3 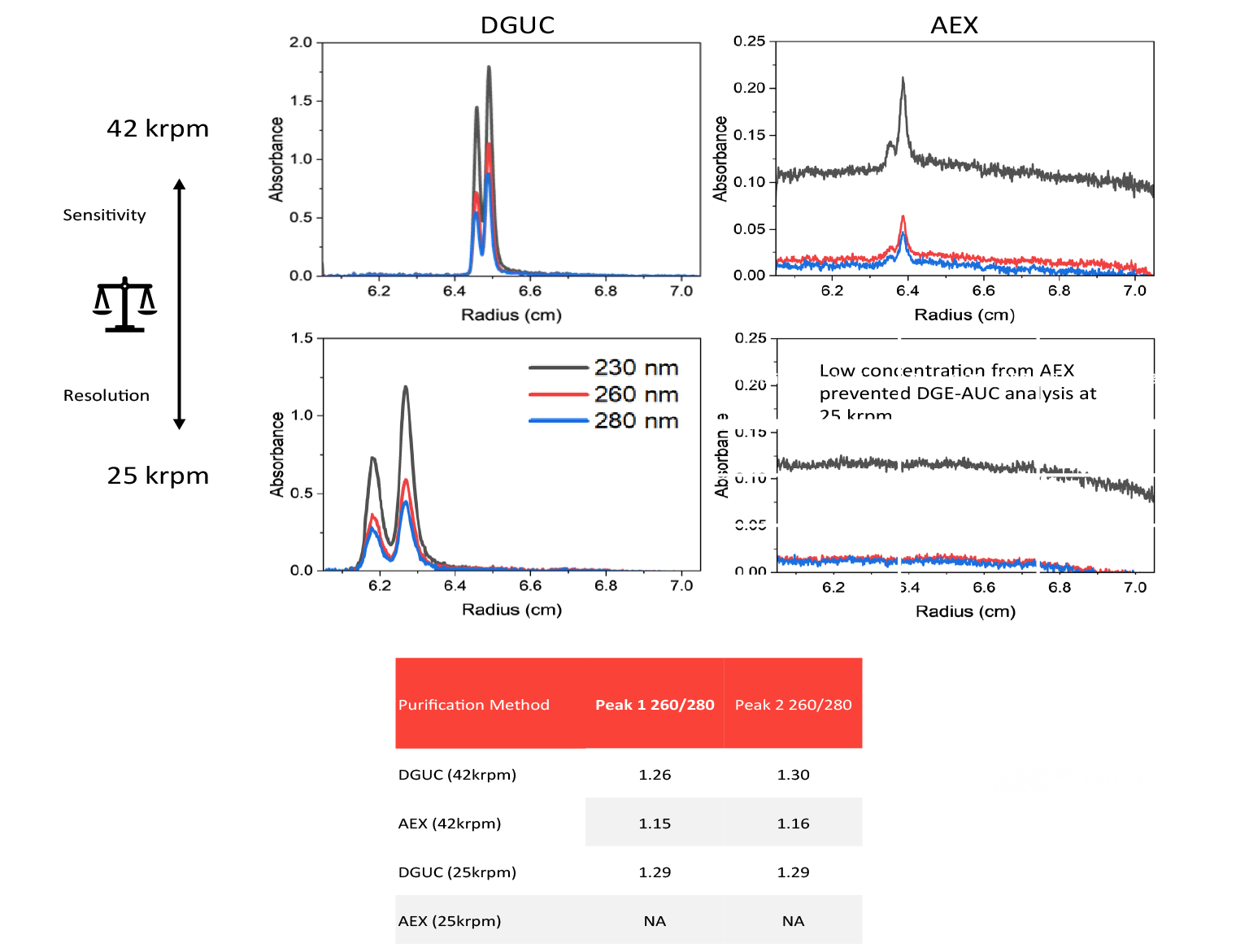 DGE-AUC characterization of vector purified using DGUC versus AEX. , higher intensity peaks were observed at 42 krpm while near baseline resolution was achieved by dropping the speed to 25 krpm for the DGUC-purified sample. Using DGE-AUC, two peaks were observed in each sample, and the 260/ 280 ratios were around 1.3, within the same range as full particles.
DGE-AUC characterization of vector purified using DGUC versus AEX. , higher intensity peaks were observed at 42 krpm while near baseline resolution was achieved by dropping the speed to 25 krpm for the DGUC-purified sample. Using DGE-AUC, two peaks were observed in each sample, and the 260/ 280 ratios were around 1.3, within the same range as full particles.
Optimizing DGUC
The protocol for DGUC previously discussed is a rapid swinging-bucket protocol, which takes around 1.5 h to complete in the SW 41 Ti. In this protocol, the result is a step gradient rather than a continuous gradient.
To improve a workflow with DGUC ( Figure 4 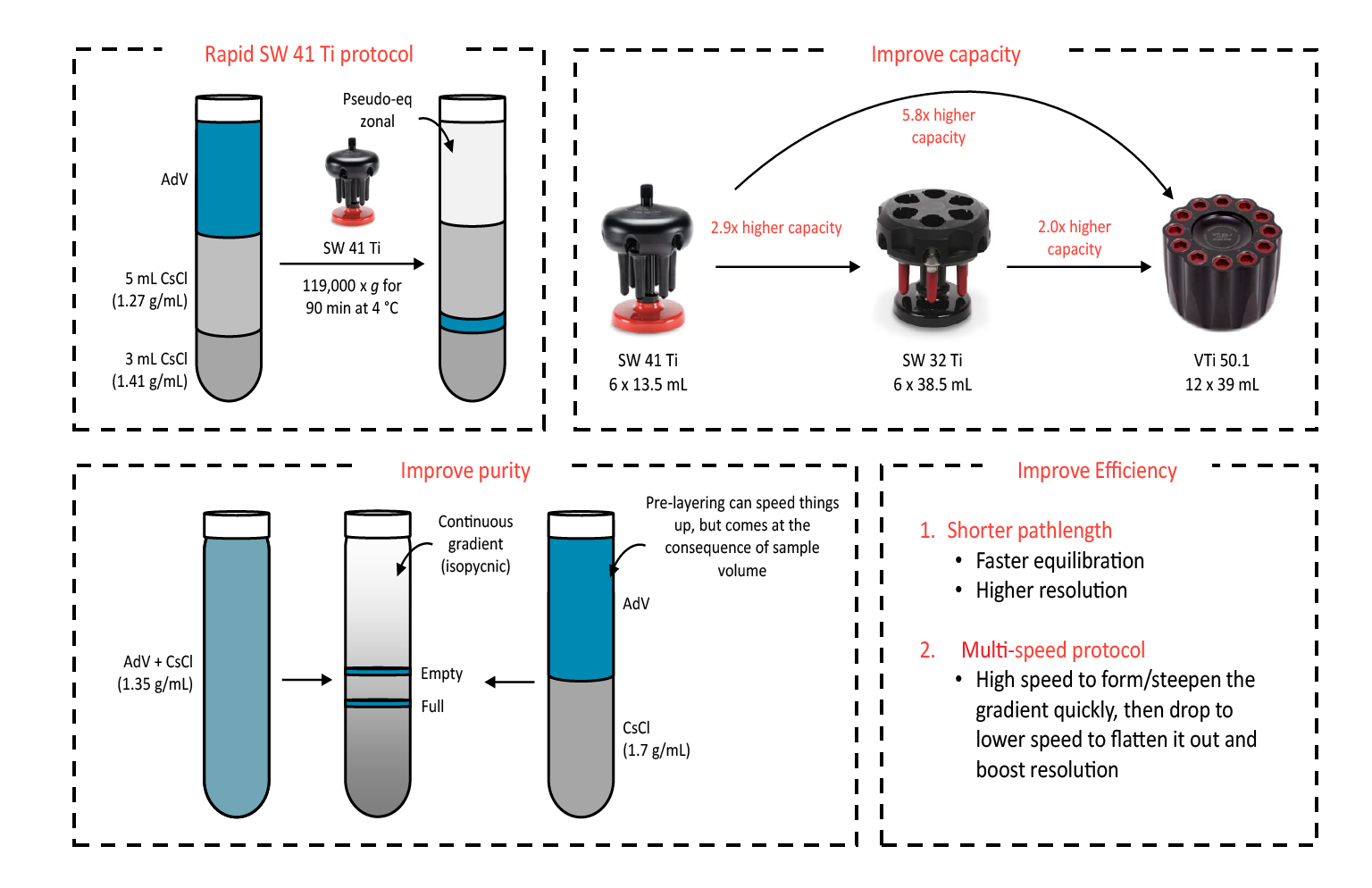 Optimization of DGUC through logical and predictable changes. ), the first aspect to consider is improving capacity. By switching to another swinging-bucket option, the SW 32 Ti, a 2.9-fold higher capacity can be achieved. In addition, by switching to Beckman Coulter’s newest vertical rotor, the VTI 50.1, an almost 6-fold increase in capacity can be further achieved.
Optimization of DGUC through logical and predictable changes. ), the first aspect to consider is improving capacity. By switching to another swinging-bucket option, the SW 32 Ti, a 2.9-fold higher capacity can be achieved. In addition, by switching to Beckman Coulter’s newest vertical rotor, the VTI 50.1, an almost 6-fold increase in capacity can be further achieved.
Another approach to improve workflow is to improve purity via continuous/linear density gradients. In contrast to the equilibrium-zonal purification using the SW 41 Ti protocol, formation of continuous gradients provides enhanced resolution. CsCl can be added directly to clarified lysate as a solid or it can also be layered with the sample on top to significantly reduce run time. However, there is usually a trade-off with sample volume in this case.
Other methods of improving DGUC efficiency include using a shorter pathlength, which allows for faster equilibration and higher resolution. This means that the vertical rotor is the best option for density gradient separations. Multi-speed protocols can also be implemented to reduce the time required to reach equilibrium.
Beckman Coulter Life Sciences offers a wide range of ultracentrifugation tubes from 2–100 mL, with the 39 mL tubes being the most popular option. Most tubes are available in a variety of formats including open top, Quick-Seal (permanent seal) and OptiSeal (plug seal). Different tube materials including polypropylene and UltraClear are also available.
Translation insight
In a case study comparing DGUC and AEX, a single run in a low-capacity swinging-bucket rotor could generate over 1.09x1013 viral particles for adenovirus. Using this AEX purification protocol, 110 L of cell culture would be needed to achieve the same yield. DGUC offers a high yield and concentration of functional particles while minimizing other contaminants. ( Figure 5 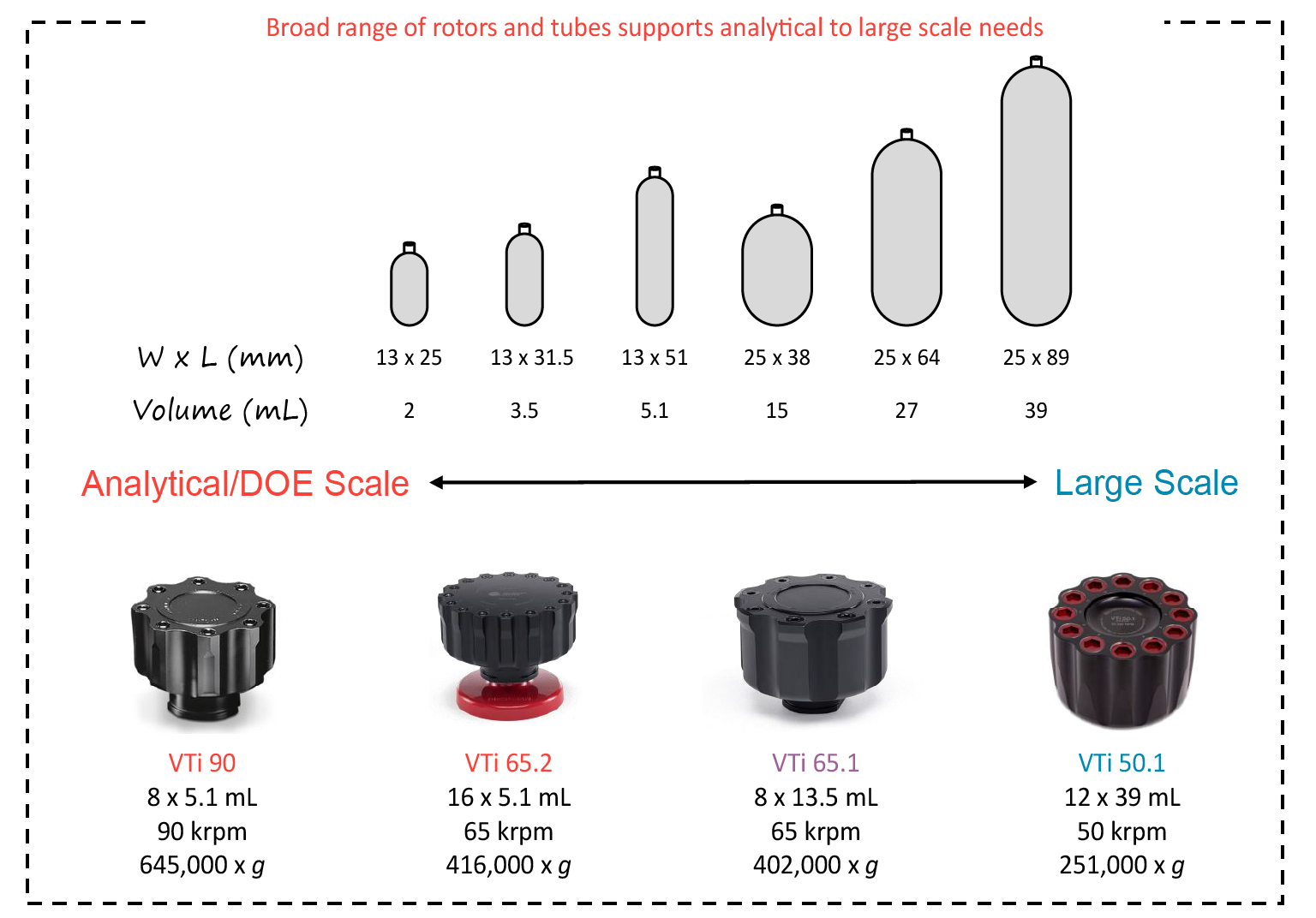 Scaling up and scaling down DGUC. ) This process is most efficient in a vertical rotor, which are available in a broad range of sizes to support analytical or Design of Experiment (DoE) needs as well as large-scale preparative workflows.
Scaling up and scaling down DGUC. ) This process is most efficient in a vertical rotor, which are available in a broad range of sizes to support analytical or Design of Experiment (DoE) needs as well as large-scale preparative workflows.
Q&A
What are the main considerations for changing from the swinging-bucket or fixed-angle to a vertical rotor?
SS: Swinging bucket has the longest pathlength, while the vertical rotor has the shortest. The fixed-angle rotors are somewhere in the middle. The short pathlength allows you to reach equilibrium significantly faster if you are running a density gradient.
In addition, our VTi 50.1 rotor has a nominal capacity of 468 mL, making it the largest vertical rotor on the market by about 50%. If you are doing step or continuous gradients on a large scale, this is the best option.
The caveat with vertical rotors is that they are only good at isopycnic density gradient. For labs with a variety of different applications including gradients, pelleting, and flotation, a fixed-angle rotor can provide the additional versatility.
Can vertical rotors be used for iodixanol gradients, and what other uses do they have?
SS: We have a significant number of customers who do use iodixanol and vertical rotors, at different scales. In this case, it does not matter if it is a step or a continuous gradient. Either one should be amenable to a vertical rotor, which has the highest efficiency for those types of purifications.
For vertical rotors, density gradients are their primary use and they are by far the best option. Fixed angles are best for versatility. The swinging bucket is the best choice if you plan on doing rate zonal experiments, which is a pelleting experiment through a gradient. The long pathlength is good for high resolution in those cases.
Have you ever tried adding CsCl in clarified lysate, and then DGUC without layering the two CsCl gradients?
SS: This is something that we have explored through a different collaboration and it should be possible. You would need to make sure that you have a sufficient concentration of your viral vector to ensure you can see the band at the end, although it may be possible to use fractionation instead of extracting bands.
What do you suggest using for the polishing step after the ultracentrifugation?
SS: The removal of CsCl can be done in a variety of ways, dependent on your needs and what volume you are working at.
At the lower scale, spin concentrators are very common. Our sister company Pall offers some that we have used in the lab ourselves. There are also de-salting columns, and at a larger scale, tangential flow filtration is another popular option for polishing.
Affiliation
Shawn Sternisha
Senior Field Applications Scientist,
Biotechnology Business Unit,
Beckman Coulter Life Sciences
Authorship & Conflict of Interest
Contributions: The named author takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author has no conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Intellectual property: This document contains confidential and proprietary information owned by Beckman Coulter, Inc. Any disclosure, use, copying or distribution of this information without the express written consent of Beckman Coulter, Inc. is strictly prohibited. Beckman Coulter makes no warranties of any kind whatsoever express or implied, with respect to this protocol, including but not limited to warranties of fitness for a particular purpose or merchantability or that the protocol is non-infringing. All warranties are expressly disclaimed. Your use of the method is solely at your own risk, without recourse to Beckman Coulter. Not intended or validated for use in the diagnosis of disease or other conditions. This protocol is for demonstration only and is not validated by Beckman Coulter. 2022-GBL-EN-100336-v1 (v1.1)
Regulatory/trademark statements: All products and services identified, unless noted as for in-vitro diagnostic (IVD) use, are for research use and not intended or validated for use in the diagnosis of disease or other conditions. ©2022 Beckman Coulter, Inc. Beckman Coulter, the stylized logo, and the Beckman Coulter product and service marks mentioned herein are trademarks or registered trademarks of Beckman Coulter, Inc. in the United States and other countries. All other trademarks are the property of their respective owners.
Attribution: Copyright © 2022 Beckman Coulter, Inc. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source:This article is a transcript of a webinar, which can be found here.
Webinar recorded: Sep 13 2022; Revised manuscript received: Oct 17 2022; Publication date: Oct 20 2022.

.png)