Walking on thin ice: controlled freezing & thawing of pharmaceutical active molecules
Cell & Gene Therapy Insights 2022; 8(11), 1457–1464
DOI: 10.18609/cgti.2022.212
Cryopreservation of active pharmaceutical ingredients, cells, or tissues is fundamental to maintain the quality of the respective product. Ice formation, re-crystallization, and other phenomena that occur during the freezing and thawing process can cause significant damage. These ice-induced injuries can be minimized by appropriate molecule design and the use of chemical cryoprotectants or bio-substances in combination with suitable equipment and physical conditions during the freezing and thawing process. It is important to adapt both freezing and thawing processes to the characteristics of the protein or active pharmaceutical ingredient that requires a cold chain to maintain its biological and chemical stability during storage and shipment. Controlled freezing and thawing facilitates consistent product quality during the fluid management processes involved in manufacturing. By preventing damage to active ingredients, the supply of medications is optimized.
The pharmaceutical industry produces highly effective, precise medications to treat important diseases, including cancers, autoimmune diseases, and rare genetic disorders. Patients around the world rely on the development, production, and supply of therapies to treat these conditions.
Stable supply chains and transportation pathways facilitate the logistics required to store and transport medicine, but cannot always guarantee that a product will not be altered until it is administered to a patient. In most cases, therefore, a product is frozen to optimize cell viability and preserve its chemical properties and molecular structure.
Controlling the freeze/thaw process
For small molecules, especially amorphous pharmaceuticals, it is crucial to maintain their chemical stability and to avoid chemical degradation or hydrolysis reactions. It is well known that proteins can become unstable when exposed to ambient or room temperature for a certain period of time, leading to their degradation or loss of functionality, which can subsequently affect their efficacy as a treatment [1]Shalaev E, Soper A, Zeitler JA, et al. Freezing of Aqueous Solutions and Chemical Stability of Amorphous Pharmaceuticals: Water Clusters Hypothesis. J. Pharm. Sci. 2019; 108, 36–49. [2]Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm. Dev. Technol. 2007; 12, 505–523. .
Particularly sensitive are parenterals containing cells, such as cell therapies and blood transfusions, as well as tissues and organs [3]Murray KA, Gibson MI. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022; 6, 579–593.. Active and viable cells comprise between 60–95 % water and dissolved molecules. The movement of molecules, including water molecules, into or out of a cell through selectively permeable pores in the cell membrane, is a continuous process needed to keep the cell alive and maintain its functionality [4]Benga G. Water transport in red blood cell membranes. Prog. Biophys. Mol. Biol. 1988; 51, 193–245.. Therefore, water cannot be removed from cells prior to their freezing, as this would disrupt osmosis, and the cells could shrivel or burst and consequently die.
The rapid cooling of intracellular water can lead to ice formation in cells, which can cause major damage. Extracellular ice crystals, on the other hand, can not only lead to the mechanical injury of cells but also to osmotic or ionic stress. These stresses can be managed by the appropriate use of cryoprotective agents (CPAs) added to a biological specimen prior to freezing and by applying optimal cooling rates [3]Murray KA, Gibson MI. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022; 6, 579–593. [5]Chang T, Zhao G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. (Weinh) 2021; 8, 2002425. [6]Tan M, Mei J, Xie J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021; 11, 68.. Cryobiology as a field was born in 1949, with Polge and colleagues’ discovery of the cryoprotective properties of glycerol [7]Polge C, Smith AU, Parkes AS. Revival of Spermatozoa after Vitrification and Dehydration at Low Temperatures. Nature 1949; 164, 666..
As with freezing, the thawing process also presents some challenges. Due to the inevitable presence of water, extracellular ice formation is unavoidable during the freeze/thaw process. Therefore, controlling the formation and growth of ice crystals is essential during both the freezing and thawing processes if fully viable cells are to be regained following cryopreservation.
The science behind the freezing of drug substances
The principles of crystallization and ice nucleation, illustrated in FIgure 1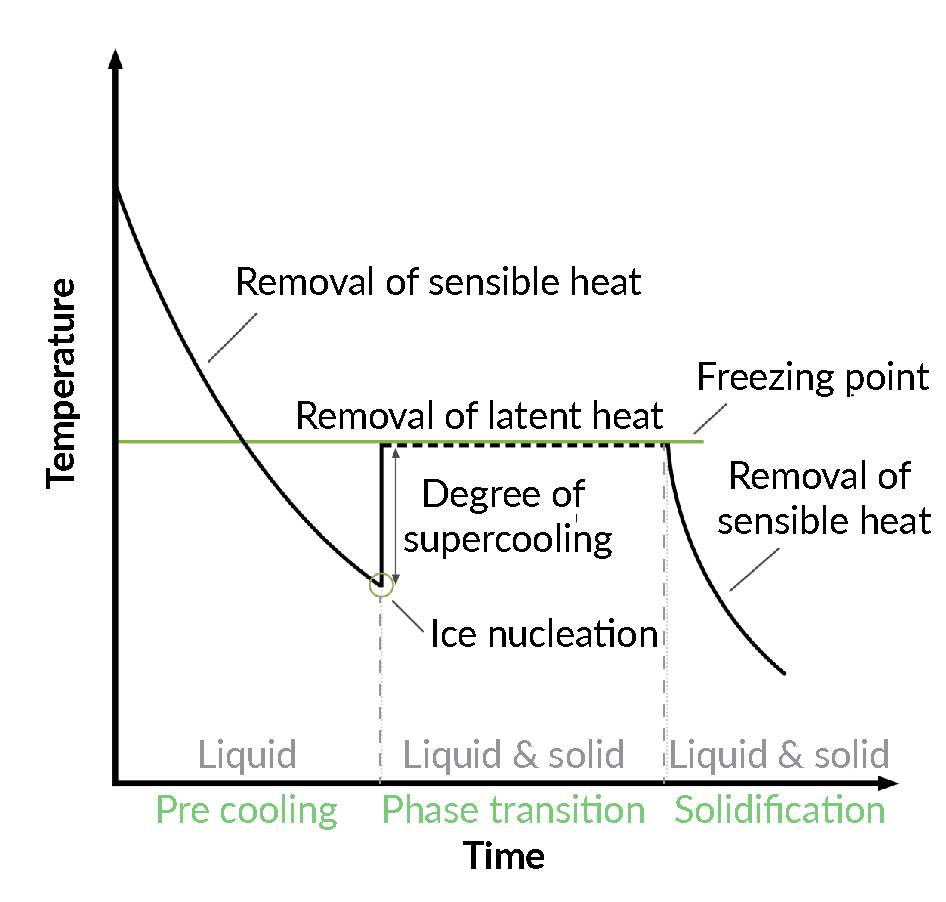 Physical processes and states of an aqueous solution during freezing., are as follows:
Physical processes and states of an aqueous solution during freezing., are as follows:
As a liquid is cooled down, the latent heat of solidification (or sensitive heat) is removed until ice begins to nucleate. During ice nucleation, the molecules of the liquid collide, aggregate, and form clusters. Nucleation can only occur once the temperature has dropped below the equilibium freezing point of the solution, thereby generating a more or less extensive degree of supercooling of the remaining liquid portion of the sample; this is a stochastic process. Ice nucleation comprises part of the phase transition, with the formation of ice crystals representing an exothermic phase change that releases heat to the environment, producing the transient warming of the sample, as shown in Figure 1 [6]Tan M, Mei J, Xie J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021; 11, 68..
If the temperature is low enough to overcome the energy barrier at the freezing point, then solidification of the liquid can proceed. The temperature range of maximum ice crystal formation in pure water is around -1°C to -5°C; this is similar for most aqueous solutions during the equilibrium freezing temperature.
Rapid freezing generates smaller and more numerous ice crystals, but this is not necessarily the best choice for every application. Therapies with a high density or large volume of cells show different heat transfer rates compared with solutions containing fewer or no cells. Slow freezing techniques are essential to guarantee sufficient cell recovery in a variety of cell types, including induced pluripotent stem cells (iPSCs) , and to increase post-thaw viability and functionality. This is especially the case for human stem cells, which are even more sensitive to ice crystal-induced injuries than animal cells or other human cells [8]Uhrig M, Ezquer F, Ezquer M. Improving Cell Recovery: Freezing and Thawing Optimization of Induced Pluripotent Stem Cells. Cells 2022; 11(5), 799..
The use of rapid freezing increases both energy and cooling equipment costs, which has an impact on total production costs [6]Tan M, Mei J, Xie J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021; 11, 68.. Consequently, for each product group, an ideal freeze and thaw ramp should be developed, with a focus on robustness, replicability, and ideally scalability.
The role of bio-inspired design & cryoprotective agents
There are various options to secure the quality and efficacy and hence the safety of a product during the freeze-thaw process. One is ‘bio-inspired’ design, which mimics anti-icing strategies found in biological systems. This can include special structures that envelop cells with so-called nanocoats, which shield cells from the mechanical damage caused by extracellular ice crystals during the processes of freezing and thawing. Encapsulation by biocompatible hydrogels has a similar effect and can also prevent devitrification effects during thawing [5]Chang T, Zhao G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. (Weinh) 2021; 8, 2002425.(devitrification is the process by which the structure of a glassy, vitreous substance changes into a crystalline solid).
Another strategy to avoid ice-induced injuries or malfunctions is the use of chemical molecules or compounds as CPAs [3]Murray KA, Gibson MI. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022; 6, 579–593.. Commonly used CPAs include dimethyl sulfoxide (DMSO) and glycerol. However, both can have negative impacts on biological materials or even result in adverse effects in patients, e.g., due to toxicity. In some cases, such permeating CPAs, usually small molecules, must be removed. This is an additional and time-consuming step following the thawing process, with a high potential for osmotic stress or even osmotic shock. Non-permeating agents, such as sugars and some polymers, can be non-toxic, but do not prevent intracellular ice formation [3]Murray KA, Gibson MI. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022; 6, 579–593. [5]Chang T, Zhao G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. (Weinh) 2021; 8, 2002425.. However, the level of CPA toxicity is dependent on the CPA concentration, the temperature, and the exposure time.
Ice-binding proteins (IBPs) have some potential as alternatives to CPAs; these include ice-nucleating proteins and antifreeze proteins. In general, IBPs alter the formation of ice crystals by attaching to the surface of ice crystals [5]Chang T, Zhao G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. (Weinh) 2021; 8, 2002425.. The presence of IBPs can improve the efficiency of freezing [6]Tan M, Mei J, Xie J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021; 11, 68..
Current commercially available cryopreservation technologies do not allow cells to be stored frozen while attached to tissue culture plastic. Cells cryopreserved with DMSO-based CPAs, for example, must be thawed from a cell bank, followed by several days of cell culture, prior to being transferred to microtiter cell culture plates [9]Congdon T, Tomas R, Fischer B. Cryopreserved Thaw and Use Cell Plates: Introducing CryoShield and the RoSS.pFTU for Cell Monolayer Cryopreservation: Application Note. SUS (2022). Congdon T, Tomas R, Fischer B. Cryopreserved Thaw and Use Cell Plates: Introducing CryoShield and the RoSS.pFTU for Cell Monolayer Cryopreservation: Application Note. SUS (2022). . This process produces large numbers of dead and detached cells, which can affect experimental results; it is also time-consuming. New, innovative cell-based assays can optimize and accelerate the discovery of pharmaceutical active compounds and discovery of cell signalling and disease pathways. Innovative proprietary pre-plated cryoprotectant technologies can successfully cryopreserve cells in adherent formats [10]Tomás RMF, Bissoyi A, Congdon TR, Gibson MI. Assay-ready Cryopreserved Cell Monolayers Enabled by Macromolecular Cryoprotectants. Biomacromol. 2022; 23, 3948–3959.. This technology, with assay-ready cell banks, can reduce the time-to-experiment by up to 90% and costs by up to 40%. Controlled freezing of cryopreserved cell monolayers can reduce the time required to perform tests to 24 h, without the need for CPAs such as DMSO [9]Congdon T, Tomas R, Fischer B. Cryopreserved Thaw and Use Cell Plates: Introducing CryoShield and the RoSS.pFTU for Cell Monolayer Cryopreservation: Application Note. SUS (2022). Congdon T, Tomas R, Fischer B. Cryopreserved Thaw and Use Cell Plates: Introducing CryoShield and the RoSS.pFTU for Cell Monolayer Cryopreservation: Application Note. SUS (2022). .
Considerable effort and resources have been invested in cryogenic research, to optimize freezing agents, their concentration, and their molecular design, and to determine optimal freezing rates and temperatures.
Thawing methods
As with freezing rates, thawing techniques can have a major impact on the processability, quality, and efficacy of a drug, and different products require different methods. The thawing method can often be overlooked during process development, which can lead to prolonged throughput times, low yields, or even different product behaviors [11]Grabarek AD, Jiskoot W, Hawe A, Pike-Overzet K, Menzen T. Forced degradation of cell-based medicinal products guided by flow imaging microscopy: Explorative studies with Jurkat cells. Eur. J. Pharm. Biopharm. 2021; 167, 38–47. such as aggregation or heterogenization, with consequences such as major deviations or batch rejects.
For the thawing of aqueous solutions, a critical temperature zone is -160°C to -15°C. Within this range small ice crystals can easily transform into large ones [5]Chang T, Zhao G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. (Weinh) 2021; 8, 2002425.. Warming rates and temperature curves during thawing impact the process of re-crystallization. It is of great importance for frozen products such as vaccines, cell therapies, and other biopharmaceuticals that quality, efficacy, and patient safety are maintained throughout the processes of freezing, storage, transport, and thawing, until the products are administered.
“In general, proteins, such as monoclonal antibodies, tend to form aggregates upon freezing and are somewhat unstable. Slow (passive) thawing can even intensify this instability.” [12]Jain K, Salamat-Miller N, Taylor K. Freeze-thaw characterization process to minimize aggregation and enable drug product manufacturing of protein based therapeutics. Sci. Rep. 2021; 11, 11332..
Passive thawing can be carried out either at room temperature (20±5°C) or in a cooled environment, for example at 5±3°C [11]Grabarek AD, Jiskoot W, Hawe A, Pike-Overzet K, Menzen T. Forced degradation of cell-based medicinal products guided by flow imaging microscopy: Explorative studies with Jurkat cells. Eur. J. Pharm. Biopharm. 2021; 167, 38–47.. A quicker and more controlled thawing method is via immersion in a water bath at a minimum of 25°C, dry automated thawers in clinical settings or through the use of a plate-based freeze/thaw platform system (Figure 2 A plate-based freeze and thaw platform system from Single Use Support to freeze to -80°C within a few hours.). The latter offers the most control, as it is possible to define standardized and automated protocols (Figure 3
A plate-based freeze and thaw platform system from Single Use Support to freeze to -80°C within a few hours.). The latter offers the most control, as it is possible to define standardized and automated protocols (Figure 3 Automated and controlled thawing of pharmaceutical active molecules upon customized recipes.) to secure reproducibility. Plate-based platforms for controlled freeze and thaw processes also offer the most flexibility in terms of volumes and the choice of primary packaging to be thawed. Furthermore, plate-based freezing can help to avoid primary packaging becoming wet, which can often lead to handling issues. It has also been shown that homogenization during thawing maximizes heat transfer and simultaneously minimizes the risk of inhomogeneities that can result from prolonged exposure to high solute concentrations and buried amino acids [12]Jain K, Salamat-Miller N, Taylor K. Freeze-thaw characterization process to minimize aggregation and enable drug product manufacturing of protein based therapeutics. Sci. Rep. 2021; 11, 11332..
Automated and controlled thawing of pharmaceutical active molecules upon customized recipes.) to secure reproducibility. Plate-based platforms for controlled freeze and thaw processes also offer the most flexibility in terms of volumes and the choice of primary packaging to be thawed. Furthermore, plate-based freezing can help to avoid primary packaging becoming wet, which can often lead to handling issues. It has also been shown that homogenization during thawing maximizes heat transfer and simultaneously minimizes the risk of inhomogeneities that can result from prolonged exposure to high solute concentrations and buried amino acids [12]Jain K, Salamat-Miller N, Taylor K. Freeze-thaw characterization process to minimize aggregation and enable drug product manufacturing of protein based therapeutics. Sci. Rep. 2021; 11, 11332..
Optimizing the process of thawing cryopreserved cells
When working with cells, such as pluripotent stem cells, the recommended approach is to optimize the thawing process by accelerating it. Slow rates of thawing can potentially harm cells and cause ice-induced injuries by prolonged exposure to too high extracellular concentrations of CPAs [8]Uhrig M, Ezquer F, Ezquer M. Improving Cell Recovery: Freezing and Thawing Optimization of Induced Pluripotent Stem Cells. Cells 2022; 11(5), 799.. Optimizing the thawing process has been shown to reduce the overall culture time of iPSCs from three weeks to between four and seven days. Otherwise, it could take up to three weeks [9]Congdon T, Tomas R, Fischer B. Cryopreserved Thaw and Use Cell Plates: Introducing CryoShield and the RoSS.pFTU for Cell Monolayer Cryopreservation: Application Note. SUS (2022). . It should be noted, however, that fast thawing is not necessarily associated with improved post-thaw recovery [13]Kilbride P, Meneghel J, Creasey G et al. Automated dry thawing of cryopreserved haematopoietic cells is not adversely influenced by cryostorage time, patient age or gender. PLoS One 2021; 15, e0240310..
Water baths, bead baths, and dry baths are widely used for warming cryovials, cryobags or well plates containing cells. A more controlled option is the use of plate-based freeze/thaw systems, such as RoSS.pFTU from Single Use Support to perform controlled freezing rate in different scales. These can be preconditioned to avoid the prolonged thawing times that can result in decreased cell viability. This type of system is fully controllable, the temperature can be monitored, and relevant data are archived according to current good practice regulations. With automated plate-based thawing technology, individual thawing processes can be programmed to ensure
optimized warming.
A Translation insight
The cryopreservation and thawing of cells is being explored to ensure the continuous research, development, and supply of biopharmaceuticals and advanced therapies. Although the history of CPAs goes back to the late 1940s, modern molecular design, new approaches to the use of synthetic and biologically inspired CPAs, and controllable, scalable equipment with standardizable freezing and thawing processes are improving the efficiency and reliability of cell therapies and biomolecules. Platform systems that enable controlled cryogenic applications contribute to high levels of cell protection throughout the cold chain. Automation plays a major role in fostering advanced freeze and thaw processes, to enable standardized and reproducible procedures. This not only reduces operational errors but also leads to an efficient and reliable approach to cold chain management. Scalable and controlled freezing and thawing, tailored to product characteristics, can pave the way for further improvements in
cryopreservation in the future.
Biography
Dr Barbara M. Fischer holds a PhD in Biology and is Process Consultant at Single Use Support. In this role, she is working together with established pharmaceutical manufacturers and start-up companies to find the most suitable solution to sustain the product quality through its journey ensuring optimized utilization of resources. Barbara has in-depth experience in low bioburden and aseptic GMP manufacturing of liquid and powder formulations from downstream processing to fill finish. Previously she held technical leadership positions supporting process development, tech transfer, manufacturing and process validation. As project manager she was involved in technical transfers and implementation of complex technical equipment.
References
1. Shalaev E, Soper A, Zeitler JA, et al. Freezing of Aqueous Solutions and Chemical Stability of Amorphous Pharmaceuticals: Water Clusters Hypothesis. J. Pharm. Sci. 2019; 108, 36–49. Crossref
2. Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm. Dev. Technol. 2007; 12, 505–523. Crossref
3. Murray KA, Gibson MI. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022; 6, 579–593. Crossref
4. Benga G. Water transport in red blood cell membranes. Prog. Biophys. Mol. Biol. 1988; 51, 193–245. Crossref
5. Chang T, Zhao G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. (Weinh) 2021; 8, 2002425. Crossref
6. Tan M, Mei J, Xie J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021; 11, 68. Crossref
7. Polge C, Smith AU, Parkes AS. Revival of Spermatozoa after Vitrification and Dehydration at Low Temperatures. Nature 1949; 164, 666. Crossref
8. Uhrig M, Ezquer F, Ezquer M. Improving Cell Recovery: Freezing and Thawing Optimization of Induced Pluripotent Stem Cells. Cells 2022; 11(5), 799. Crossref
9. Congdon T, Tomas R, Fischer B. Cryopreserved Thaw and Use Cell Plates: Introducing CryoShield and the RoSS.pFTU for Cell Monolayer Cryopreservation: Application Note. SUS (2022). Crossref
10. Tomás RMF, Bissoyi A, Congdon TR, Gibson MI. Assay-ready Cryopreserved Cell Monolayers Enabled by Macromolecular Cryoprotectants. Biomacromol. 2022; 23, 3948–3959. Crossref
11. Grabarek AD, Jiskoot W, Hawe A, Pike-Overzet K, Menzen T. Forced degradation of cell-based medicinal products guided by flow imaging microscopy: Explorative studies with Jurkat cells. Eur. J. Pharm. Biopharm. 2021; 167, 38–47. Crossref
12. Jain K, Salamat-Miller N, Taylor K. Freeze-thaw characterization process to minimize aggregation and enable drug product manufacturing of protein based therapeutics. Sci. Rep. 2021; 11, 11332. Crossref
13. Kilbride P, Meneghel J, Creasey G et al. Automated dry thawing of cryopreserved haematopoietic cells is not adversely influenced by cryostorage time, patient age or gender. PLoS One 2021; 15, e0240310. Crossref
Affiliation
Barbara Fischer
Process Consultant,
Single Use Support, Kufstein, Austria
Authorship & Conflict of Interest
Contributions: The named author takes responsibility for the integrity of the work as a whole, and has given her approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author has no conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Single Use Support. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Aug 31 2022; Revised manuscript received: Nov 23 2022; Publication date: Dec 14 2022.

