RCA-free production of third generation adenoviral vectors
Cell & Gene Therapy Insights 2022; 8(11), 1469–1474
DOI: 10.18609/cgti.2022.215
Adenoviruses (Ad) have a long history as DNA-transfer vehicles in various medical applications including in vivo gene therapy [1]. In this context, the favorable safety profile of Ad has been considered a major benefit, since the lack of integration into the host cell genome eliminates potential risks associated with insertional mutagenesis. This is combined with a comparatively high packaging capacity for foreign DNA, which is relevant in projects where the therapeutic expression cassette is too large for other gene therapy vectors such as adeno-associated viruses. Further advantages include high infectivity, high-titer virus production, excellent stability, and a broad tissue tropism.
Generations of adenoviral vectors
For gene therapy applications, with the exception of oncolytic viral therapies, Ad vectors which cannot further multiply after they have transferred the therapeutic gene into the patient’s cells are used. Such replication-incompetent Ad vectors avoid the risk of triggering potential pathogenic processes and an unwanted immune response, as might be the case for wildtype Ad [2]Lochmüller H, Jani A, Huard J et al. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (delta E1 + delta E3) during multiple passages in 293 cells. Hum. Gene Ther. 1994; 5, 1485–1491. [3]Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Progr. Neurobiol. 1998; 55, 399–432.. The first generation of replication-incompetent Ad, called Ad∆E1 vectors, carry a deletion of the E1 region of the adenoviral genome which encodes proteins that are crucial for virus replication. In addition, the adenoviral E3 gene is usually also deleted in such vectors to create additional space for the insertion of a transgene. Although the cargo capacity of traditional first-generation adenoviral vectors (~8 kb) is already significantly larger than that of adeno-associated viruses (~4.7 kb), this is not sufficient for projects in which the transfer vector delivers large coding sequences, like the huntingtin (9.4 kb) or dystrophin (11 kb) genes, multiple genes, long cis-regulatory elements or gene editing tools. Furthermore, proteins expressed from the remaining adenoviral genes in Ad∆E1 vectors can elicit immune responses in vivo [4]Yang Y, Ertl HC, Wilson JM. MHC class I-cestricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1994; 1, 433–442. that can negatively affect the expression of the therapeutic protein and thus its efficacy. To address these challenges, additional regions of the Ad genome were deleted in subsequent vector generations. In the second vector generation extra deletions were inserted into the E2 and E4 region of the adenovirus genome [5]Wang Q, Finer MH. Second-generation adenovirus vectors. Nat. Med. 1996; 2, 714–716., leading to a decreased background production of viral proteins and an increase of cargo capacity (~14 kb). To further improve these properties, all viral genes were finally eliminated in the third generation of adenoviral (Ad3rd) vectors, also referred to as gutless, gutted or helper-dependent adenoviral vectors [6]Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ. (Figure 1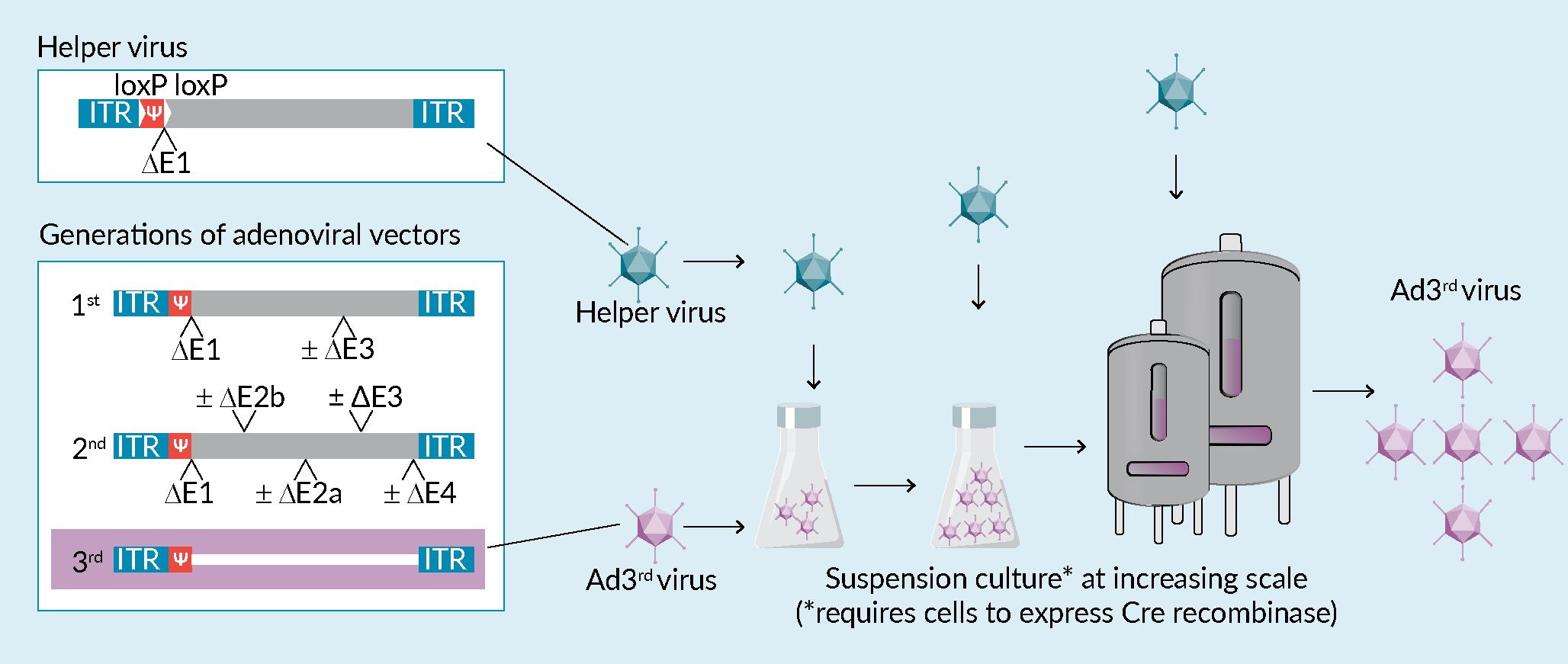 Production of Ad<sup>3rd</sup> vectors in suspension cells.The left box illustrates the genome of the helper virus and of the different generations of adenoviral vectors. The flow chart at the bottom schematically depicts the production process of Ad3rd vectors suspension cells. Production is conducted in increasingly larger volumes with cells requiring coinfection with a helper virus at each step. Note that if CAP cells are used for production they will need to express Cre recombinase in order to delete the loxP-flanked packaging signal Ψ of such helper virus. RCA-formation can occur at any step, including the production of the helper virus itself., box). These vectors combine a very high packaging capacity for foreign DNA (~36 kb) with a low immunogenicity, making them attractive vectors for in vivo gene therapy.
Production of Ad<sup>3rd</sup> vectors in suspension cells.The left box illustrates the genome of the helper virus and of the different generations of adenoviral vectors. The flow chart at the bottom schematically depicts the production process of Ad3rd vectors suspension cells. Production is conducted in increasingly larger volumes with cells requiring coinfection with a helper virus at each step. Note that if CAP cells are used for production they will need to express Cre recombinase in order to delete the loxP-flanked packaging signal Ψ of such helper virus. RCA-formation can occur at any step, including the production of the helper virus itself., box). These vectors combine a very high packaging capacity for foreign DNA (~36 kb) with a low immunogenicity, making them attractive vectors for in vivo gene therapy.
Production of Ad3rd vectors
In comparison to AdDE1 vectors, the production of Ad3rd vectors poses the challenge that all genes necessary for virus production have been removed from the virus genome and must therefore be made available in trans during virus manufacturing. AdDE1 vectors are typically produced in cell lines that carry stable insertions of the Ad E1 region in their genome, such as HEK293 [7]Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1997; 36, 59–74., HER-911 [8]Fallaux FJ, Kranenburg O, Cramer SJ et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther. 1996; 7, 215–222., PER.C6 [9]Fallaux FJ, Bout A, van der Velde I et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 1998; 9, 1909–1917. or CAP [10]Schiedner G, Hertel S, Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 200; 11, 2105–2116. cells, and thus trans-complement the missing E1 gene functions. In these cells, a primary stock of recombinant Ad is generated by transfection with a linearized plasmid vector, so that the packaged transfer vector contains the gene of interest (GOI) as well as the adenoviral genes required for virus production, except for E1. The primary Ad stock can subsequently be amplified in E1 trans-complementing cells to produce sufficient material for medical applications [11]Wissing S, Faust N, Scheer N. Overview: Manufacturing Adenoviral Vectors at Large Scale. Genetic Eng. & Biotechnol. News 2021; 41, 35..
In contrast, Ad3rd vectors lack any viral protein-coding genes, so that E1 trans-complementing cells transfected with gutless vectors are unable to produce new viral particles. A simple amplification of primary viral stocks in, for example, HEK293 or CAPTM cells, as with Ad∆E1 vectors, is therefore not possible with Ad3rd vectors. Instead, such vectors are typically produced in the presence of an E1-deficient adenoviral helper virus that provides the required viral gene functions for packaging of the Ad3rd vectors, hence their designation as helper-dependent adenoviral vectors [6]Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.. However, this comes with another challenge, namely to separate the therapeutic adenovirus from the replication-competent helper virus so that the latter does not contaminate the final drug preparation. This can be achieved by a combination of two approaches [6]Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.:
- The packaging signal Ψ of the helper virus is flanked by loxP sites and thus excised by Cre recombinase, expressed in the cells used for virus production. Consequently, the helper virus genome is not packaged into viral particles but is still able to trans-complement replication and encapsidation of the Ad3rd genome.
- Certain differences between the helper virus and the Ad3rd vector can be exploited to remove the former. For example, the size of the helper virus and the Ad3rd genome differ sufficiently from each other, allowing for the separation of the respective viral particles. The two viruses also differ in various physicochemical properties, such as charge density and hydrophobicity, which enables the removal of the helper virus by scalable chromatographic methods.
Risk of RCA formation during standard Ad3rd production processes
In addition to the challenge of separating the helper virus, there is another issue when using HEK293 cells for vector production – the risk of emerging replication-competent adenoviruses (RCA) [6]Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.. The rationale for using HEK293 is that for safety reasons an E1-deficient helper virus is normally utilized and its missing E1 function must be provided by the packaging cells. This constellation, however, holds the potential for homologous recombination between the helper virus and the Ad sequences integrated into the HEK293 cells genome, which can lead to RCA formation [12]Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA. 1996; 93, 13565–13570.. For the reasons outlined above, the presence of RCAs in clinical batches is highly undesirable and limited by the FDA to less than one RCA in 3×1010 viral particles for such preparations. To minimize the risk of RCA formation during the production of Ad3rd vectors in HEK293 cells, the length of the initial helper virus genome is increased to the upper packaging limit by inserting stuffer sequences. After homologous recombination this limit is exceeded and the virus genome can no longer be packaged efficiently into viral particles [6]Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ.. Nevertheless, clinical preparations must be tested for RCA and discarded if the acceptable limit is exceeded, which can be associated with high costs and significant time expenditures.
RCA-free Ad3rd production in CAP TM cells
In the interest of product safety, it is clearly preferable to avoid homologous recombination during Ad3rd vector production instead of preventing the packaging of the recombinant DNA into virus particles. This can in fact be achieved when specific cell lines, such as the CAP cells, are used for virus production. CAP cells were isolated during a routine amniocentesis and subsequently immortalized with Ad E1 gene functions. Importantly, the transforming plasmid for CAP cells was designed in a way so that it lacks sequence overlap with typically used adenoviral vectors [10]Schiedner G, Hertel S, Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 200; 11, 2105–2116., and various studies demonstrated that no detectable RCA were generated by CAP cells amongst 5×1010 Ad viral particles [11]Wissing S, Faust N, Scheer N. Overview: Manufacturing Adenoviral Vectors at Large Scale. Genetic Eng. & Biotechnol. News 2021; 41, 35. [13]Scheer N. Manufacturing of RCA-free adenoviral vectors. Cell Gene Ther. Insights 2022; 08, 351. . As described above, Ad3rd vector production is ideally done in CAP cells which in parallel express the Cre recombinase. This can be achieved by stably integrating the corresponding gene into customized CAP cells. After transfecting the linearized plasmid with the gene transfer vector into such customized CAP cells, the cells are co-infected with a E1-deficient helper virus containing a loxP-flanked packaging signal Y. Cre-mediated excision of Y prevents packaging of the helper virus genome into viral particles, whereas Ad3rd vectors are effectively produced. Subsequent serial rounds of co-infections with helper virus and Ad3rd vectors are performed to increase Ad3rd vector yields (Figure 1, flow chart), followed by quality control to obtain a well-defined master virus seed. Importantly, amplification steps and final batch productions can be readily scaled-up to required volumes due to reliable growth of CAP suspension cells to high cell densities in various bioreactor formats even in serum- and animal component-free media. Together with their traceable cell line history and availability of fully characterized GMP Master cell banks, CAP cells represent an ideal platform for high titer Ad3rd vector production in regulated biopharmaceutical environments.
References
1. Wold WSM, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013; 13, 421–433. Crossref
2. Lochmüller H, Jani A, Huard J et al. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (delta E1 + delta E3) during multiple passages in 293 cells. Hum. Gene Ther. 1994; 5, 1485–1491. Crossref
3. Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Progr. Neurobiol. 1998; 55, 399–432. Crossref
4. Yang Y, Ertl HC, Wilson JM. MHC class I-cestricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1994; 1, 433–442. Crossref
5. Wang Q, Finer MH. Second-generation adenovirus vectors. Nat. Med. 1996; 2, 714–716. Crossref
6. Palmer DJ, Ng P. Methods for the Production of Helper-Dependent Adenoviral Vectors. In: Gene Therapy Protocols: Production and In Vivo Applications of Gene Transfer Vectors. (Editors: Le Doux JM) 2008, 33–54, Humana Press, Totowa, NJ. Crossref
7. Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1997; 36, 59–74. Crossref
8. Fallaux FJ, Kranenburg O, Cramer SJ et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther. 1996; 7, 215–222. Crossref
9. Fallaux FJ, Bout A, van der Velde I et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 1998; 9, 1909–1917. Crossref
10. Schiedner G, Hertel S, Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 200; 11, 2105–2116. Crossref
11. Wissing S, Faust N, Scheer N. Overview: Manufacturing Adenoviral Vectors at Large Scale. Genetic Eng. & Biotechnol. News 2021; 41, 35. Crossref
12. Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA. 1996; 93, 13565–13570. Crossref
13. Scheer N. Manufacturing of RCA-free adenoviral vectors. Cell Gene Ther. Insights 2022; 08, 351. Crossref
Affiliations
Nico Scheer
CEVEC, now part of Cytiva,
Cologne, Germany
and
FH Aachen, University of Applied Sciences,
Germany
Helmut Kewes
CEVEC, now part of Cytiva,
Cologne, Germany
Ulrich Kettling
CEVEC, now part of Cytiva,
Cologne, Germany
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: We would like to thank Vanessa Kress for her valuable input to this article.
Disclosure and potential conflicts of interest: The authors are employees of CEVEC, now part of Cytiva, which is the owner of the CAP cell line and the CAP AdTM technology and trademark.
Funding declaration: Except for the employment by CEVEC, the authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Cytiva. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Sponsored article; externally peer reviewed.
Submitted for peer review: Aug 31 2022; Revised manuscript received: Nov 23 2022; Publication date: Dec 15 2022

