Enabling GMP-ready cell sorting for cell and gene therapy manufacturing
Cell & Gene Therapy Insights 2022; 8(10), 1339–1344
DOI: 10.18609/cgti.2022.194
David McCall, Editor, Cell & Gene Therapy Insights, talks to Aditi Singh, Sony Biotechnology
Cell sorting has become a key point of focus for cell therapy process developers in recent times. Can you first frame for us why this is the case? What are the drivers behind this quest for improvement?
AS: Cell and gene therapies are revolutionary technologies, and the space is rapidly evolving. Several leading pharma companies are advancing their manufacturing workflows to come up with more potent, adoptive cellular therapies that provide long-term remission in patients.
One of the commonalities between the manufacturing strategies rendering better therapeutic potency, particularly in the field of chimeric antigen receptor (CAR) T-cell therapy, is that most strategies utilize immune cell subtypes for CAR engineering. In this case, the manufacturers specifically isolate young, less differentiated cells for developing therapies, which are known to have longer-term persistence. Isolation of these rare cells from a heterogeneous population of cells present in patients’ blood requires selection based on multiple parameters. This is where a flow cytometry-based cell sorting technology adds value, because it can enable isolation of cells based on multiple immunological markers. Also, the higher purity of selection enables improvement in manufacturing reproducibility, especially in the autologous segment, where starting material is highly variable.
Can you go into more depth on the current cell sorting isolation toolkit? What are some of the specific pros and cons of the various options available?
AS: The two commonly used cell isolation technologies currently utilized in the cell therapy manufacturing space are magnetic cell separation, where cells are isolated using magnetic beads attached to specific antibodies, and fluorescence-based cell isolation, where cells are stained with fluorochrome antibodies and sorted. While the magnetic separation technologies can be faster and have higher throughput, they can only isolate cells based on one or two parameters. Flow cytometry-based cell sorting technologies are desirable because they offer selection based on multiple parameters (up to ten) simultaneously, and the isolated cells are highly pure. While flow cytometry-based cell sorting methods are more sophisticated and sensitive, they offer lower throughput and are relatively expensive compared to magnetic-bead based separation. Also, the lack of a fully GMP-ready flow cytometry-based cell isolation system limited the adoption of these technologies for utilization in clinical-grade manufacturing.
The CGX10 Cell Isolation System is one of a number of recent technological innovations in this particular space. What differ-entiates it? What specific benefits does it offer?
AS: The CGX10 Cell Isolation System is a novel system which enables GMP-ready cell sorting for clinical-grade cell and gene therapy manufacturing. With its unique and proprietary microfluidic chip and built-in temperature control cabinet, the CGX10 instrument enables fully closed, high speed, high purity, multiparametric, fluorescence-based cell sorting for all kinds of cell and gene therapy manufacturing workflows.
The CGX10 Cell Isolation System is unique because the entire cell sorting process occurs inside the microfluidic chip, which is fully closed, and the entire system (instrument, single-use kit, and software) is designed with the needs of GMP-grade cell and gene therapy manufacturing in mind. This means that the user can take their therapies from process development to clinical or commercial manufacturing smoothly.
At Sony, with the CGX10, we can offer GMP readiness at every step:
- The CGX10 instrument is manufactured in an ISO 9001-certified facility. It’s an automated, standalone closed system with a compact design, specifically catering to the GMP environment.
- All of the consumables that go with the CGX10 system are single-use kits manufactured in an ISO 13485-certified facility. They have undergone biocompatibility evaluation as per ISO 10993 guidelines. We also offer all the necessary regulatory and quality support documentation with each of our CGX10-related products to enable clinical/commercial manufacturing.
- To ensure safety and security of patients’ electronic records, the CGX10 software offers three different modes of operation (roles):
- Administrator Mode;
- A more open Process Development Mode, enabling the user to create and optimize the protocol, and;
- A more controlled Standard Operation Mode, allowing the user to simply execute the previously created protocols. In the Standard Operation Mode, all the operations are recorded and compliant with 21 CFR Part 11 guidelines.
Can you share any data on the CGX10 Cell Isolation System in practical application, particularly regarding its relative performance in terms of speed, purity, and cell viability/quality?
AS: The CGX10 system is designed to allow workflow flexibility, providing offering different speed and purity options for different workflows. The instrument offers an Enrichment Mode, which can sort cells at a high speed of up to 100,000 events per second (eps), and a Purity Mode, which can deliver a purity level of about 97% at up to 15,000 eps. There are two additional sort modes: Yield Mode improves yield by maximizing sort efficiency, while Custom Purity Mode offers adjustable balance between purity and yield (sort efficiency) whereby the user can select one of the ten purity levels available.
The microfluidic-based hydrodynamic sorting ensures that the cells are gently processed and retain high viability. To validate this, we compared the viability of cells from three samples which were sorted using the CGX10 system with the viability of cells sorted using traditional cuvette-based sorters (Figure 1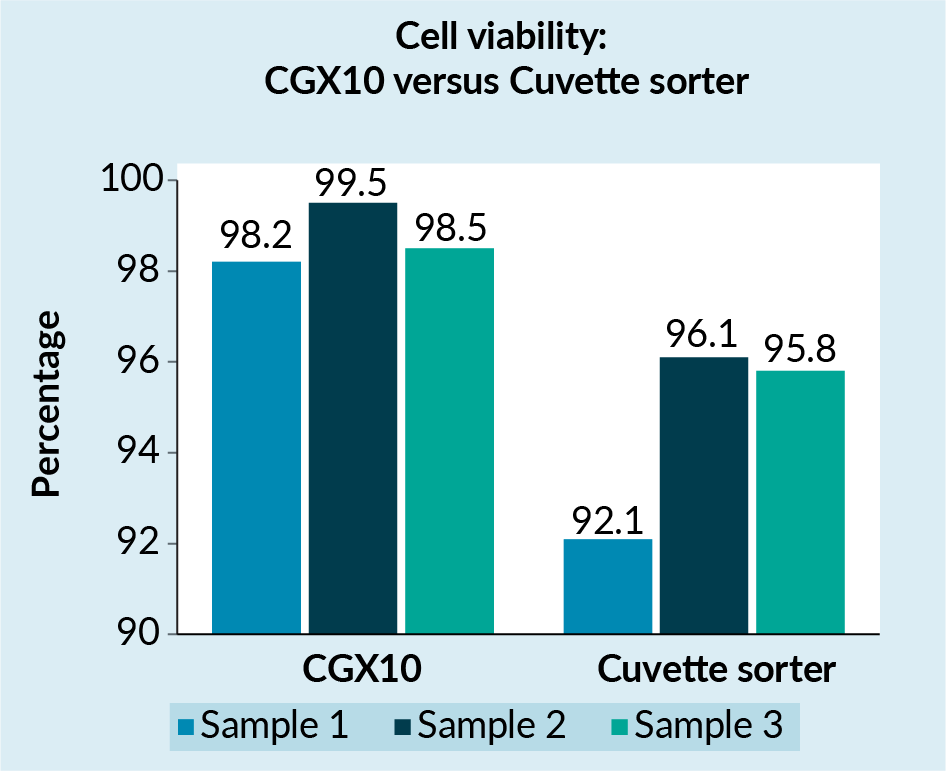 ). We found the viability of cells sorted using the CGX10 system to be 3–5% higher than the viability of cells sorted using traditional cuvette based-sorters.
). We found the viability of cells sorted using the CGX10 system to be 3–5% higher than the viability of cells sorted using traditional cuvette based-sorters.
What specific cell types and sources has the instrument been tested on and what have been your observations from these studies?
AS: Owing to Sony’s legacy of innovation in the field of intuitive system design, optics, and automation, the previous-generation Sony cells sorters such as the MA900, SH800S, and FX500 have been used in pioneering cell therapy studies conducted in various well-known laboratories or institutions worldwide. The CGX10 retains the versatility of its predecessors and has been successfully deployed in workflows utilizing various kinds of immune cells, such as T cells, regulatory T cells (Tregs), and NK Cells.
We have also successfully tested the usability of the CGX10 in isolating tumor infiltrating lymphocytes (TILs) and different stem cells, including iPSC-derived cardiomyocytes and hematopoietic stem cells (HSCs).
The results from these studies consistently demonstrate that the CGX10 can isolate cell fractions present in either low or abundant levels at high throughput. The results also indicate that the CGX10 can be easily integrated with closed upstream and downstream CAR-T process steps, with the potential to be integrated into various alternative immunotherapy and other cell and gene therapy manufacturing pipelines.
How have you ensured ease of operation?
AS: The CGX10 system is designed to ensure relatively hands-off cell sorting by offering automation at multiple levels from setup to analysis, such as automated chip positioning and optical axis adjustment, automated sample line cleaning and end-of-sort detection, etc.
However, one of the key outstanding features of this instrument, which has been widely appreciated by our early users, is the automatic clog detection and recovery technology. During sorting, any clogs that may be formed during sample flow are automatically detected by the system through the scatter-based detection of the sorted cells. The clogs are then automatically disintegrated by controlling the fluidics, through multiple adjustments as needed. This ensures robust operation over long sorting times and promotes walk-away operation.
What will be some next steps in the further development and optimization of this technology by Sony?
AS: At this time, Sony Biotechnology is focusing on partnering with different cell therapy process development and manufacturing organizations to generate proof of concept clinical-grade, multiparametric cell isolation data. These studies involve integrating the CGX10 system in multiple cell and gene therapy manufacturing workflows, including various stem cell therapies (hematopoietic stem cells, mesenchymal stem cells, induced pluripotent stem cells, genes edited with CRISPR/Cas9, etc.), ensuring interoperability with various pre- and post-processing manufacturing steps.
In the future, we would like to employ more advanced computational capabilities such as machine learning to make our algorithms more robust for varied sample and sort protocols, and carefully consider user feedback to make incremental improvements to operability.
Finally, we are always committed to engaging with life scientists at all levels to help them create the next innovation and, as we say at Sony, generate Kando!
Affiliation
Aditi Singh
Sony Biotechnology, Inc.
Authorship & conflict of interest
Contributions: The named author takes responsibility for the integrity of the work as a whole, and has given her approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author has no conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2023 Sony Biotechnology, Inc. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited.
Interview conducted: Oct 21 2022; Revised manuscript received: Nov 3 2022; Publication date: Feb 8 2023.


