The importance of material selection in the differentiation of monocytes into dendritic cells
Cell Gene Therapy Insights 2018; 4(8), 509-522.
10.18609/cgti.2018.052
Submitted for Peer Review: 31 Jan 2018 Published: 6 Nov 2018
INTRODUCTION
Cell therapy is an emerging market with the potential to shift the paradigm for how diseases are treated. In recent years, the ‘cancer immunotherapy’ subset of the market has seen rapid growth garnered from the efficacy of the often personalized treatment approach. Named ‘Breakthrough of the Year’ by Science magazine in 2013, the immunotherapy market has started to really come to fruition in the past few years, including the recent FDA approval of Novartis’ CAR-T therapy, Kymriah™ (tisagenlecleucel) and Kite’s CAR-T therapy, Yescarta™ (axicabtagene ciloleucel) [1–3].
One of the earlier areas of research and development in immunotherapy was in the use of dendritic cell (DC)-based treatments. DCs, often considered the most potent of the antigen presenting cells (APC), play a key role in connecting the innate and adaptive immune system [4,5]. Akin to a billboard, DCs process and then present antigens on their surfaces in order to appropriately activate T cells for immune attacks. This presentation and activation step is often the missing link in an immune response to cancer as the signal is not appropriately processed by the DC when the cancer is seen as ‘self’. To tackle this, many researchers have looked to exploit the function of DCs through the culturing of monocytes, differentiation to DCs, and modifying DCs with appropriate tumor signals ex-vivo. This causes immune system stimulation upon re-infusion of activated, mature DCs (Figure 1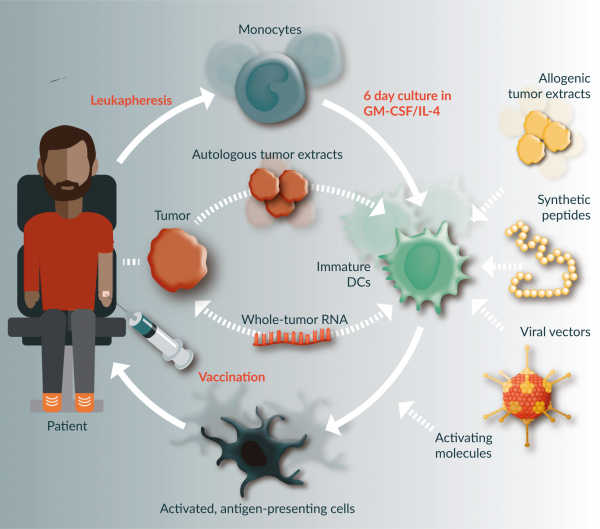
Although there are several early and late stage clinical trials leveraging this approach, there is currently only one, commercially available dendritic-based therapy in the USA [6]. Provenge® (Sipuleucel-T) was commercialized by Dendreon in 2010 and is still available for the treatment of advanced prostate cancer [7]. Recent market reports have anticipated an annual growth rate for the ‘Global Dendritic Cell & Tumor Cell Cancer Vaccine’ of 20.7% until 2030 [8].
With increasing interest in bringing cell-based products to fruition comes the increasing importance of selecting appropriate systems and materials for manufacturing. This paper will focus on reviewing material properties and important factors to consider when choosing a container for culture. With that, it also critical to consider process steps upstream (e.g., enrichment) and downstream (e.g., harvest) of culture as well as ancillary materials (e.g., medium and cytokines) when deciding on and developing a complete manufacturing process [10–12].
Current landscape of systems for monocyte to dendritic cell culture
The use of disposable materials for cell culture dates back to the 1960s. What started out as a transition from glass petri dishes to more rigid plastic containers (T-Flask), the modern landscape of culture systems is now a dynamic mixture of rigid and flexible systems that are still evolving with new material innovations [13].
Table 1 outlines a high level landscape of common culture systems for monocyte to DC culture.
| Table 1. Common Culture Systems For Monocyte to Dendritic Cell Culture. | |||
|---|---|---|---|
| Product | Company | Material Class | Type of System |
| T-Flask and Stacks | Multiple | Polystyrene | Rigid Flask |
| Evolve® | OriGen | Polyolefin | Flexible Bag |
| EXP-PAK™ | Charter Medical | Polyolefin | Flexible Bag |
| PermaLife™ | OriGen | Fluoropolymer | Flexible Bag |
| MACS® GMP Cell Differentiation Bag | Miltenyi | Polyolefin | Flexible Bag |
| VueLife® | Saint-Gobain | Fluoropolymer | Flexible Bag |
Critical material properties to consider when selecting system for cell culture
Oxygen permeability & water vapor permeability
There are many factors that impact the permeability of polymers, including, but not limited to:
- Size/physical state of penetrating molecule
- Morphology/properties of the polymer
- Solubility/diffusivity of the permeant
- Presence of fillers, humidity and plasticizers
Permeability of gasses, including water vapor, is arguably the most critical property when selecting a closed culture system. This is because the cells will depend on permeation through the material of the system in order to maintain appropriate levels of oxygen (and carbon dioxide) and on a sufficient barrier to limit evaporation – all critical to the overall metabolic function of the cells. Humidified incubators are often required for cell culture to mitigate water loss from the cell environment (Figure 2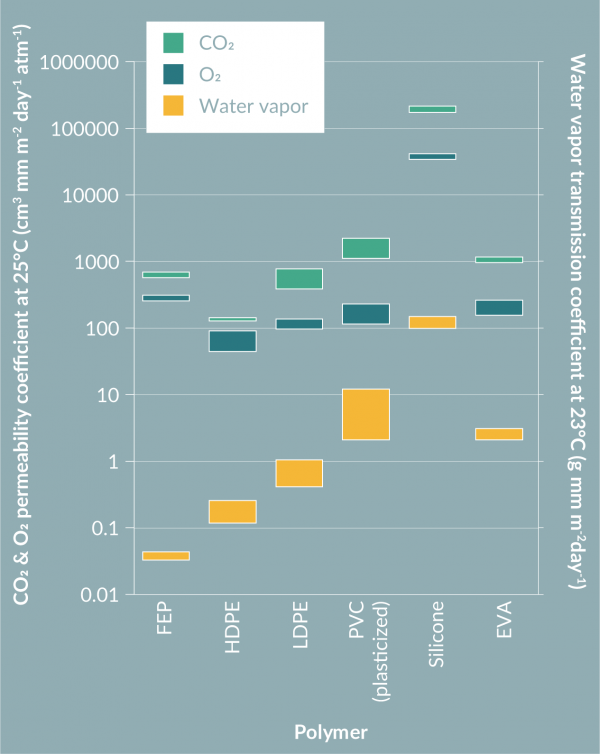

Transparency
Transparency is a physical property that is used to describe the ability of light to pass through a material. ‘Light’ can refer to a multitude of wavelengths in the ultraviolet, visible, and infrared spectrum.
Benefits of transparency to specific wavelengths depend on desired application. Some examples include, but are not limited to:
Visible light transparency (400–700 nm)
- Optical microscope imaging
- Visual inspection of culture (e.g., for contamination or pH change)
- Fluorescence microscope imaging
UV-A light transparency (320–400 nm)
- Photopheresis
- Fluorescence microscope imaging
In any of these cases, the ability to leverage the transparency properties of the bag rather than taking samples or changing containers reduces labor, manipulation of the culture environment and, ultimately, chance for contamination.
Extractables & leachables
Extractables and leachables are terms used to describe migrating compounds in various conditions.
Extractables
Organic and inorganic chemical entities that can migrate from the contact surface under aggressive conditions. Aggressive conditions could include:
- Elevated temperature
- Extended contact time
- Aggressive solvents
Extractables have the potential to leach into a product under conditions of storage and use [17].
Leachables
Organic and inorganic chemical entities that migrate from the contact surface under application-specific or ‘working’ conditions [17]. These are generally considered a subset of extractables but not all leachables will be identified by typical extraction testing (Figure 5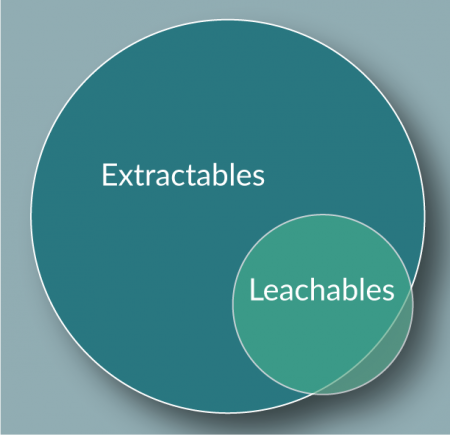
As leachables are extremely specific to a particular process, there is no generic test that can be performed for identification. In most cases, extractable testing with multiple solvents, alongside sufficiently sensitive analytical tools, can be used as a general proxy for migrating compounds. Some of the most common methods are shown in Table 2.
In general, it is imperative to quantitatively identify and understand potentially harmful impurities that may migrate from a plastic device and risk negatively impacting the cells during the culture period. In 2013, Amgen experienced, first-hand, the impact even small amounts of migrating compounds can have. At the time, Amgen investigated the effect of Irgafos® 168, a common antioxidant in polyolefins, on the growth of several Chinese Hamster Ovary (CHO) cell lines [18]. Eventually, and after thorough investigation of options, Amgen ended up working closely with their supplier to optimize the starting amount of Irgafos in the starting material which, as a result, lowered the leaching compound concentration and ultimately its detrimental effects [18].
Beyond their immediate impact on cell culture, there is also a risk of migrating compounds contaminating the downstream steps of drug manufacturing, especially in the manufacturing of cell-based therapeutics when there is no final filtration step.
| Table 2. Overview of Common Extractables and Leachables analytical methods [17]. | |
|---|---|
| Method | Description |
| Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP/AES) | Quantitative analysis for metal ions |
| Direct-injection Gas Chromatography / Mass Spectrometry (DI GC/MS) | Semi-quantitative analysis for semi-volatile and volatile organic compounds |
| Liquid Chromatography / Ultraviolet / Mass Spectrometry (LC/UV/MS) | Semi-quantitative analysis for non-volatile organic compounds |
| Ion Chromatography (IC) | Quantitative analysis for ions |
| Total Organic Carbon (TOC) | Concentration of total organic carbon analysis |
Summary
Material properties like permeability, transparency, and extractables are all critical to consider when selecting a system for cell culture. With that said, there are several other material and non-material properties to bear in mind when choosing an appropriate solution. These can include, but are not limited to:
- Closed system options
- Size and shape configuration
- Type of tubing, ports, and connectors
- Sterility and shelf life
In the subsequent sections of this report, properties of fluorinated ethylene propylene (FEP), a common culture material, will be outlined alongside culture data in an effort to empirically understand the relationship between key material properties and performance in cell culture.
DETAILED MATERIAL PROPERTIES OF FLUORINATED ETHYLENE PROPYLENE
FEP is a fully fluorinated fluoropolymer with several inherent material properties that make it suitable for many cell therapy applications, including cell culture. In this section, data on FEP is presented to align with the aforementioned critical properties for cell culture systems.
All properties data provided are based on measurements of 5 mil (0.127 mm) film, a common thickness used in FEP culture containers. For permeability properties, Ethylene vinyl acetate (EVA) film was also tested to help establish a point of comparison given it is also commonly used in cell culture applications. In these cases, measurements were performed on 8 mil (0.203 mm) film, an ordinary thickness for the EVA culture container.
Oxygen permeability
Oxygen transmission rate (OTR) was measured using a MOCON OxTran 220 OTR Analyzer following ASTM D3985 at both 25°C and 37°C (Table 3).
| Table 3. Oxygen Permeability of FEP and EVA. | ||
|---|---|---|
| Temperature (°C) | OTR (cc/(m²·day·atm) | |
| FEP | EVA | |
| 25 | ~2,000 | ~1,500 |
| 37 | ~2,900 | ~2,600 |
| EVA: Ethylene vinyl acetate; FEP: Fluorinated ethylene propylene; OTR: Oxygen transmission rate. | ||
The oxygen permeability of FEP allows for a substantial transmission of oxygen, sufficient for metabolism in many cell culture processes.
Water vapor permeability
Water vapor transmission rate (WVTR) was measured using a MOCON Permatran W700 Water Vapor Analyzer following ASTM F1249 (Table 4).
| Table 4. Water Vapor Permeability of FEP and EVA. | |||
|---|---|---|---|
| Temperature (°C) | Humidity | WVTR (g/(m²·day·atm) | |
| FEP | EVA | ||
| 37.8 | 100% | ~0.5 | ~5 |
| EVA: Ethylene vinyl acetate; FEP: Fluorinated ethylene propylene; WVTR: Water vapor transmission rate. | |||
While FEP is substantially permeable to oxygen, it is an effectively strong barrier to water vapor, usually eliminating the need for humidification for prevention of significant water loss in the incubator. This can be seen from the data in table 5. The data shows average water loss over a total of 14 days for six water-filled FEP bags in a 40°C non-humidified oven. (Table 5).
| Table 5. Water loss in FEP bag. | |||
|---|---|---|---|
| Temperature (°C) | # of days | Average water loss (%) | Standard deviation (±%) |
| 40 | 14 | 0.21% | 0.01% |
| FEP: Fluorinated ethylene propylene. | |||
Extractables
Unlike most polymers, FEP film is extruded without the use of any additives (e.g., antioxidants, plasticizers, processing aids, etc.). As a fully fluorinated polymer it has very high inherent stability, and there are no modifiers or other content to leach out in water or other solvents. Consequently, extractables are typically at or below detection limits (Table 6).
| Table 6. Summary of Extractables Profile in FEP. | |||
|---|---|---|---|
| Analyte type | Analytical method | Extractables in water | Extractables 70% ethanol/30% water by volume |
| Metals | ICP/AES | Ca (0.01 mg/l) | Not detected |
| Semi-volatile and volatile compounds | Direct injection GC/MS | Not detected | Not detected |
| Semi-volatile and non-volatile compounds | LC/UV/MS | Not detected | Not detected |
| Ions | IC | Not detected | Not detected |
| Organic carbon | TOC | 0.30 mg/l (0.00010 mg/cm2) | N/A |
| GC/MS: Gas chromatography mass spectrometry; IC: Ion Chromatography; ICP/AES: Inductively coupled plasma atomic emission spectroscopy; LOD: Limit of detection TOC: Total organic carbon. | |||
Results are based on the pooled analysis of two, sterile 2PF-0290 VueLife® FEP Bags. Extraction was performed in either water or 70% ethanol/30% water by volume at 70°C for 24 h at a 3 cm2:1 ml extraction ratio.
Transparency
FEP light transmission was measured on a Perkin Elmer UV-Vis-NIR spectrophotometer.
Light transparency of FEP is among the highest for plastics, allowing for easy viewing through the film itself. This is important for morphology characteristics as well as highlighting drastic changes in cell environment through markers such as phenol red (Table 7).
| Table 7. Transparency Properties of FEP. | |
|---|---|
| Wavelength type | Transmission (%) |
| Visible | 94.80% |
| UV-A | 93.10% |
CULTURE & DIFFERENTIATION OF MONOCYTES TO DENDRITIC CELLS UTILIZING FEP CULTURE SYSTEM
Method
Monocyte enrichment
The Elutra (Terumo) cell processing system was used as per manufacturer’s instructions. In short, HBSS (Lonza) supplemented with 1% HSA (CSL) was connected to the media line, 0.9% sodium chloride (Baxter) was connected to the secondary media line and fresh volunteer apheresis (Key Biologics, TN) was attached to the sample input line of the Elutra disposable kit.
The Elutra separation program was run as per manufacturer’s instructions, shown in Table 8.
| Table 8. Elutra Separation Program Details. | |||
|---|---|---|---|
| Step | Flow rate (ml/min) | Fraction volume (ml) | Centrifuge speed (rpm) |
| Fraction 1 | 37 | 900 | 2400 |
| Fraction 2 | 97.5 | 975 | 2400 |
| Fraction 3 | 103.4 | 975 | 2400 |
| Fraction 4 | 103.9 | 975 | 2400 |
| Fraction 5 | 103.9 | 250 | 0 |
| Other Settings left as default. Flow ramp 0.1 ml/min/s, centrifuge ramp 528 rpm/s and cell:media ratio 1:1. | |||
Monocyte culture/differentiation
The fraction containing the majority of monocytes was centrifuged at 800 × g for 5 min, and resuspended in complete CellGenix® GMP DC Medium (supplemented with 500 U/ml GM-CSF and 500 U/ml IL-4 [both CellGenix]). 1 million cells/ml were transferred to a VueLife® 160-C1 bag (Saint-Gobain). The contents of the bag were mixed thoroughly by rocking/inversion, then placed in a standard humidified tissue culture incubator (37°C, 5% CO2) for 7 days.
On Days 1, 3, 5 and 7 the bags were removed from the incubator, viewed under an inverted microscope, and a sample taken after thoroughly mixing. On days 1 and 3, fresh DC medium was supplemented to ensure there was no change in volume after sampling. On day 5, the cultures were supplemented with DC medium and additionally 500 ng/ml tumor necrosis factor (TNF-α, CellGenix). On day 7, the cultures were stopped and cells were collected for analysis.
Dendritic cell yield is calculated as a percentage in relation to the starting number of monocytes.
Analytics
Immediately after taking a sample, acid/base and respiratory parameters (pCO2, pO2 and pH) were measured on the Nova pHOx bioanalyzer.
A second sample was frozen prior to thawing and running lactate, glucose, glutamine and ammonium analytics on the Cedex Bioanalyzer (Roche). A complete blood count (CBC) was completed on the AC•T DIFF™ Hematology Analyzer, Beckman Coulter.
Cell count and viability were conducted on the NucleoCounter-200, Chemometec prior to running flow cytometry (FACS) samples on Gallios, Beckman Coulter with the below panels (Table 9).
Compensation and analysis was performed using FlowJo software.
| Table 9. FACS Panel Dendritic Cell Analysis. | |
|---|---|
| FL1 | HLA-DR FITC* |
| FL2 | CD66b PE |
| FL4 | 7-AAD |
| FL6 | CD83 APC# |
| FL8 | CD14 APC-Cy7 |
| *For additional markers of DC maturation in the FL1 channel, FITC-conjugated an-tibodies to CD80 and CD40 may be used. | |
| #For additional markers of DC maturation in the FL6 channel, APC-conjugated an-tibodies to CD1a and CD86 may be used. | |
Results
Cell viability & yield
The use of FEP bags for culture show comparable yields (60%), Figure 6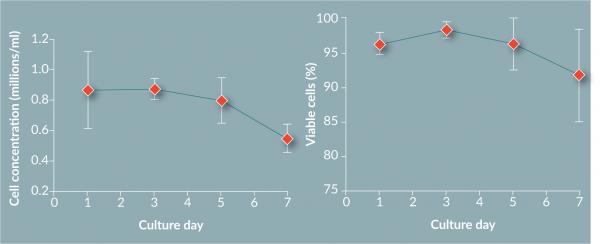
Chemistry analysis
The chemistry analysis highlights that the pH, pO2 and pCO2 levels all remained consistent during culture, maintaining equilibrium with the environment (Figure 7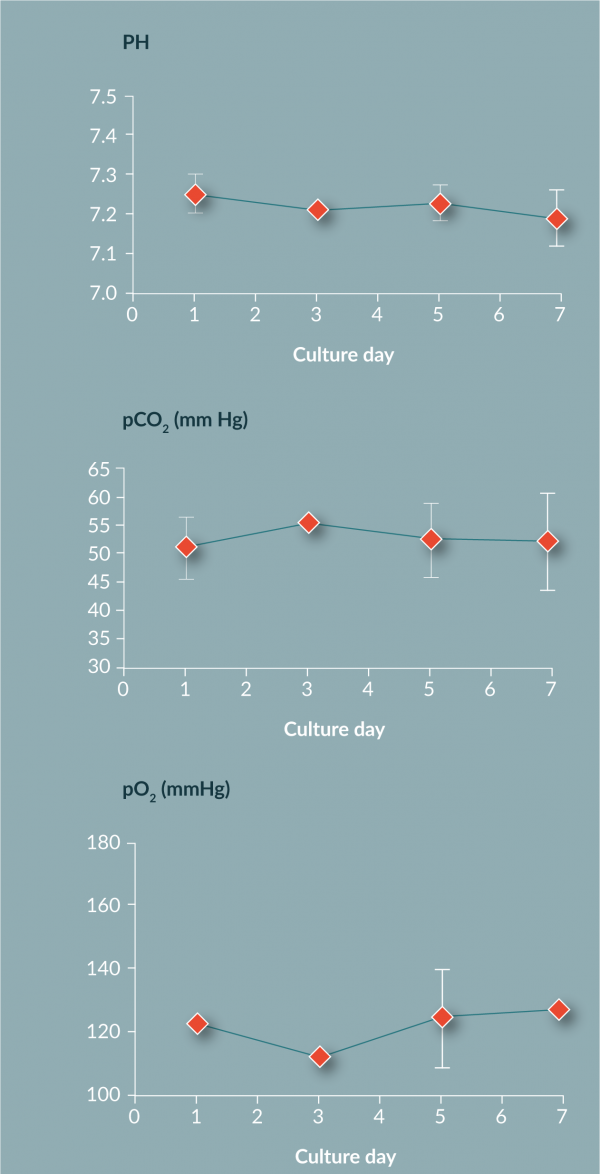
Other chemistries (lactate, ammonium, glucose, glutamine, LDH and total protein) were within expected ranges for monocyte to DC culture (Figure 8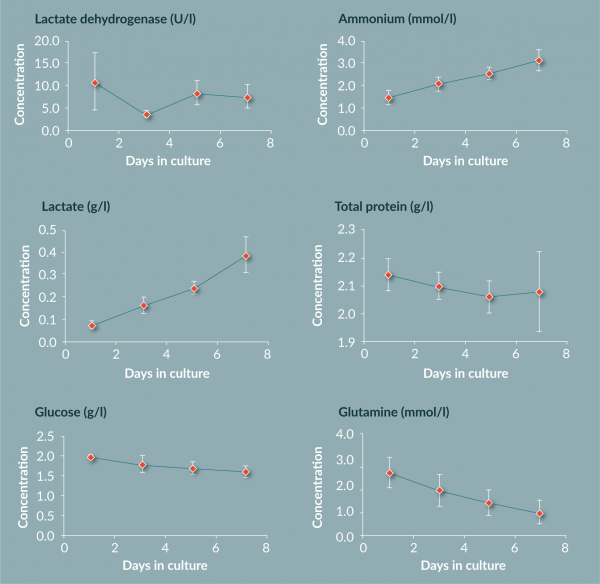
Phenotype analysis
Phenotyping by flow cytometry showed an increase in cell size and complexity (increase in forward and side scatter) during differentiation (Figure 9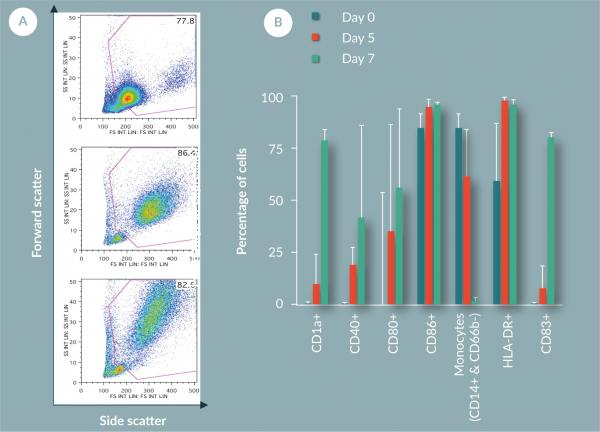
Summary
The work in this paper has shown that the use of FEP bags for the culture and differentiation of monocytes to DCs produces results in line with values from other vessels. The yield (60%) and viability (92%) are comparable to previous reported values. Chemistry analysis suggests that FEP bag properties suitably control the cell environment by providing great gas exchange as measured by pO2, pCO2 and pH. The metabolite data suggests active metabolism of cells, suggesting again the cells are in a healthy state within the FEP bag. Phenotyping of the cells shows a change over time from a large monocyte population above 75% (by CD14 marker) on day 1 to an immature DC population by day 7 (loss of CD14+ to less than 10% and greater than 75% for CD40+, CD83+, CD86+ and HLA-DR+).
CONCLUSION
When choosing a container for cell culture, there are many elements to consider beyond traditional ‘biocompatibility’. Some material properties to look for, and which have been outlined in this article include:
- Gas & water vapor permeability
- Transparency
- Extractables profile
Furthermore, there are a multitude of aspects to consider when selecting a finished culture container. These can include things such as:
- Is the container considered a close system?
- What size and shape configurations are available?
- What are the tubing, port, and connector options?
- How is the product sterilized?
- What is the product shelf life?
Based on critical material properties, VueLife®, made from FEP, was highlighted as a suitable option for the culture and differentiation of monocytes. However, given the diversity of manufacturing and cell types in the cell therapy market, there is rarely a one-size-fits-all solution for cell culture processes. Discussions with suppliers early on are critical to ensuring that the correct material is selected and design features are implemented to suit specific manufacturing needs.
Acknowledgements
We thank Dr Courtney LeBlon, Dr David O’Neill, and Dr Yajuan Jiang (all of Hitachi Chemical Advanced Therapeutics Solutions) for their help in the cell culture experiments and discussions on the results. We would also like to thank Saint-Gobain Research – Shanghai for bag extractables measurements, and CellGenix for providing the media and cytokines required for the culture experiments.
REFERENCES
1. Couzin-Frankel J. Cancer Immunotherapy. Science 2013; 342(6165): 1432–1433. CrossRef
2. Novartis Receives First Ever FDA Approval for a CAR-T Cell Therapy, Kymriah™ (CTL019), for Children and Young Adults with B-Cell ALL That Is Refractory or Has Relapsed at Least Twice. Novartis, 30 August 2017. www.novartis.com/news/media-releases/novartis-receives-first-ever-fda-approval-car-t-cell-therapy-kymriahtm-ctl019.
3. Kite’s Yescarta™ (Axicabtagene Ciloleucel) Becomes First CAR T Therapy Approved by the FDA for the Treatment of Adult Patients With Relapsed or Refractory Large B-Cell Lymphoma After Two or More Lines of Systemic Therapy. 18 October 2017. www.gilead.com/news/press-releases/2017/10/kites-yescarta-axicabtagene-ciloleucel-becomes-first-car-t-therapy-approved-by-the-fda-for-the-treatment-of-adult-patients-with-relapsed-or-refractory-large-bcell-lymphoma-after-two-or-more-lines-of-systemic-therapy.
4. Strioga MM, Felzmann T, Powell DJ Jr et al. Therapeutic dendritic cell-based cancer vaccines: the state of the art. Crit. Rev. Immunol. 2013; 33(6): 489–547. CrossRef
5. Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Current Topics in Microbiology and Immunology. Curr. Top. Microbiol. Immunol. 2006; 311(1): 17–58. CrossRef
6. Yi DH, Appel S. Current status and future perspectives of dendritic cell–based cancer immunotherapy. Scand. J. Immunol. 2013; 78(2): 167–171. CrossRef
7. FDA Approval for Sipuleucel-T. National Cancer Institute. www.cancer.gov/about-cancer/treatment/drugs/fda-sipuleucel-T.
8. Dendritic Cell and Tumor Cell Cancer Vaccines Market, 2016–2030. Research and Markets 2016. www.researchandmarkets.com/research/8pcb9s/dendritic_cell.
9. Rossini M, Rizzo P, Bononi I et al. New perspectives on diagnosis and therapy of malignant pleural mesothelioma. Front Oncol. 2018. CrossRef
10. Tuyaerts S, Aerts JL, Corthals J et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol. Immunother. 2007; 56(10): 1513–1537. CrossRef
11. Eyrich M, Schreiber SC, Rachor J et al. Development and validation of a fully GMP-compliant production process of autologous, tumor-lysate-pulsed dendritic cells. Cytotherapy 2014; 16(7): 946–964. CrossRef
12. Adamson L, Palmborg A, Svensson A et al. Development of a technology platform for large-scale clinical grade production of DC. Cytotherapy 2004; 6(4): 363–371. CrossRef
13. Niazi SK. Disposable Bioprocessing Systems. CRC Press 2012.
14. Encyclopedia of PVC volume 3 compound design and additives. Nass LI, Heiberger CA (Eds). Marcel Dekker, New York 1988.
15. Patrick SG. Practical Guide to Polyvinyl Chloride. Rapra Technology Limited, UK 2005.
16. DePalma A. Extractables and Leachables: Standardizing Approaches to Manage the Risk. BioProcess International, MA, USA 2017.
17. Hammond M, Nunn H, Rogers G, Lee H et al. Identification of a leachable compound detrimental to cell growth in single-use bioprocess containers. PDA J. Pharm. Sci. Technol. 2013; 67(2): 123–134. CrossRef
18. Guyre CA, Fisher JL, Waugh MG et al. Advantages of hydrophobic culture bags over flasks for the generation of monocyte-derived dendritic cells for clinical applications. J. Immunol. Methods 2002; 262(1–2): 85–94. CrossRef
19. Dohnal AM, Graffi S, Witt V. Comparative evaluation of techniques for the manufacturing of dendritic cell-based cancer vaccines. J. Cell. Mol. Med. 2009; 13(1): 125–135. CrossRef
20. Berger TG, Feuerstein B, Strasser E et al. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories™. J. Immuno. Methods 2002; 268(2), 131–140. CrossRef
Affiliations
Natasha Boghosian (Formerly) Saint-Gobain
Robert Pleydon Saint-Gobain
David Smith, PhD Hitachi Chemical Advanced Therapeutics Solutions

This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.