Strategies for Dealing with Donor Variability
Cell Gene Therapy Insights 2018; 4(9), 901-909.
10.18609/cgti.2018.087
The growth of cell and gene therapies has opened the doors to revolutionary new treatment options by harnessing the power of the body’s own cells (or donors cells in an allogenic setting). Cells that are highly specialised in a complex network, further modified to overcome the complexity of disease evasion. complex solutions come with complex challenges one of which is the donor variability associated to the cell source. Stem cell transplant have been a curative treatment for patients with blood cancer and other haematological disorders for over 60 years. Here we explore the challenges and strategies employed in this setting to overcome the hurdles of donor variability.
Submitted for Peer Review: Aug 24 2018 Published: Nov 20 2018
There has been years of research and huge amount of investment that has brought us to a potential tipping point in the cell and gene therapy field. Two pioneering advanced cellular therapy products have received approval and are now commercially available, leading the way in a field poised to erupt as there are currently 854 companies working to develop advanced therapies [1]. However, there are a number of big challenges to overcome if they are to have a significant impact on patients’ lives on a mass scale. Complex problems often require complex solutions, and with childhood acute lymphoblastic leukaemia and advanced lymphomas as the target, it is no surprise that the first two approved advanced cellular therapy products utilize a complicated adoptive cell transfer approach to target these diseases. However, the less of that complexity that we can introduce, the faster our research and trial processes can become. One of the easiest ways to reduce the variables and complexity, is by getting smarter in the way we select and use the starting cellular materials.
Here we explore examples of the depths of complexity from the Anthony Nolan registry and Cord Blood Programme and how we may learn from them to quickly overcome them in the cell and gene therapy setting.
Anthony Nolan has been providing cells for therapy for over 40 years. Established by Shirley Nolan in 1974 in the absence of an available donor for her son, Anthony, who was suffering from Wiskott–Aldrich syndrome, which, at the time, was a condition that only a bone marrow transplant could cure. Shirley’s vision was to establish a register of Human Leucocyte Antigen (HLA) typed individuals who would be willing to donate bone marrow to anyone in need. The primary critical attribute associated to the donor is HLA type. As a result, the fate of the graft and its success is weighted on donor selection.
Cord Blood Banking is a bit different, however: whilst maintaining the same registry mentality, its ethos is to ensure there are sufficient donors or ‘products’ to meet the needs of patients by recruiting well matched population demographics. They, in addition, provide rigorous assessment of products to avoid poor engraftment. The goal is to provide a well-defined and tested product that can guarantee quality with known variability. This is the fundamental of Quality by Design (QbD).
QbD requires the identification of a target quality product profile (TQPP). In the instance of a cord blood unit or adult cells for transplant, the profile would include features such as the ability to home to the bone marrow and the ability to repopulate the hematopoietic system in a timely fashion. Once the TQPP is sufficiently understood, a set of critical quality attributes should be identified that guarantee the product will achieve its TQPP [2].
This is very difficult for cell therapies because of the complexity of identifying characteristics of a cell population that guarantee its function. It is argued by some to be unachievable due to the intricacy and number of potential dimensions in data sets, and limited knowledge of mechanism of action. This should not deter us from thinking, in terms of best practice, and using it as a framework to guide development. In the cord setting, we have quasi-critical quality attributes such as total nucleated cell count [3] and CD34 expression [2,4,5], which correlate to functional outcome in many scenarios. However, they aren’t necessarily key determinants of function. Using them to judge the outcome of process modification would be insufficient and potentially dangerous as it may not relate to clinical function.
DONOR VARIABILITY
Looking at lessons from the Histocompatibility and Immunogenetics (H&I) field, Since the Anthony Nolan’s inception in 1974 there has been vast advancements in the field. In 1974 less than 100 HLA had been identified and therefore could be typed in order to find a tissue match. To date there are currently greater than 20 000 HLA and related alleles identified [6] with a potential of 6.9 x 1017 different combinations. The actual figure of observable variations would be much lower due to known HLA associations, which limit the total number of variations. However, this illustrates the magnitude of donor variability. There are two key lessons here:
- Donors/Patients are ‘unique’, HLA is an extreme example but for reasons discussed later an individual is the result of a myriad of interconnecting complexities and many of which could have an impact on the TQPP and therefore ‘function’ of the products;
- The variability you think you know now, could just be the tip of the iceberg.
It is essential to understand the factors of incoming product variability; every donor is different. Where selection cannot be applied it’s important to know whether the downstream process controls can compensate for the levels of variability to produce a standardized and defined end product. For example, the starting number of the target cell type will almost certainly be a critical factor. Too few cells and the process may not show an economically sensible return whilst too high could alter the growth or transformation kinetics. It may be possible to compensate for this with process controls and appropriate dosing but where the controls are not possible, pre-screening is essential to ensure the end product remains defined. If the risk of process failure can be avoided through critical evaluation of the starting material then this will save money in the longer term, as typically the starting material is a relatively small proportion of the overall process costs.
There are three ways to reduce variability that will ensure the product meets its specification prior to being provided to a patient:
- Selection
- Automation of the design space
- Rejection
SELECTION
Lessons on improving product specification can be learned from Cord Blood Banking. The Anthony Nolan Cell Therapy Centre receives between 7,000 and 10,000 cord blood units every year.
Figure 1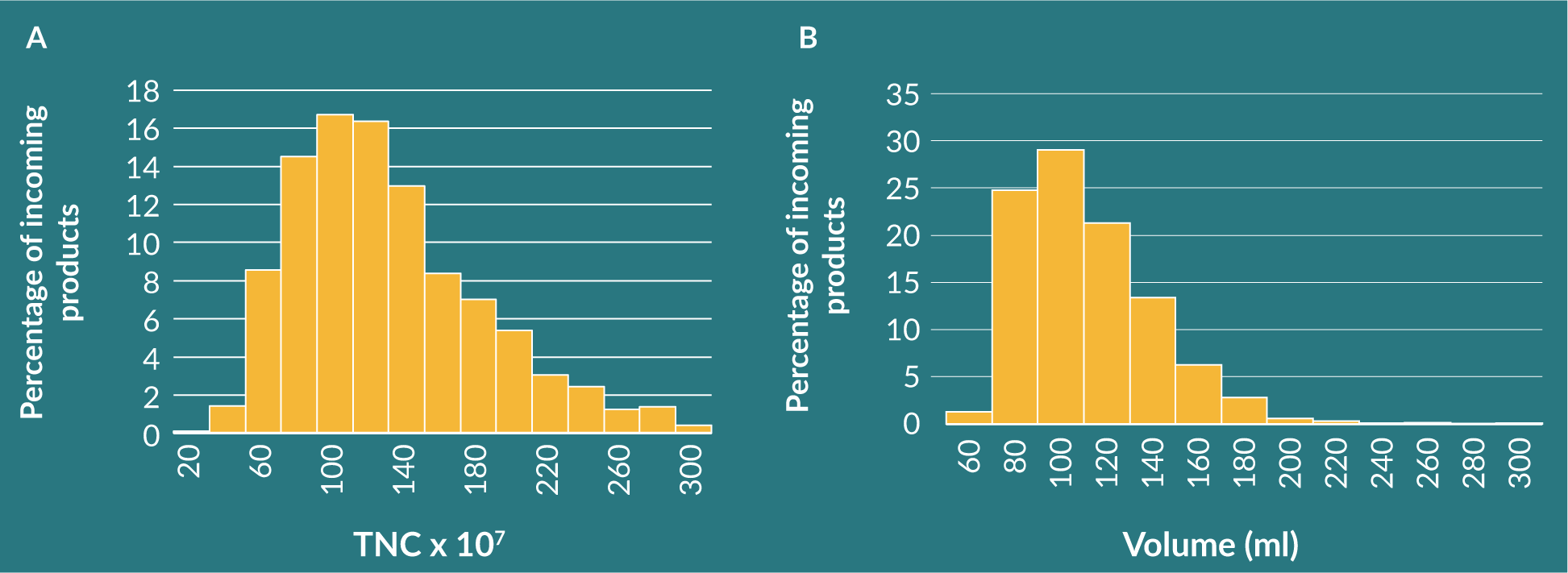
In our setting the critical quality attributes will be TNC, so we can select the donor products that will meet our needs. We can further predict which products are most likely to meet the specification through observation of donor variables such as age and ethnicity. Procurement variables are also important, such as the collection methods or the birth type, which have been demonstrated to impact the quality of the unit collected.
High stress births have increased TNC and CD34+ [7,8] cells compared to low stress births such as elective caesarean sections. The cellular signals that led the increased TNC and CD34+ cells in the high stress births could have had a further impact that means the product may respond differently. Likewise, in the adult stem cell setting, it is well known that patients fair better when a donation comes from younger donors [9]. Selecting TNC in the cord or HLA in the adult donation is potentially insufficient to qualify the TQPP as the same products (i.e., will home to the bone marrow niche and reconstitute the marrow in a timely fashion). The range of variability as observed in transplant outcome include graft failure, graft success or, on the other end of the scale, graft-versus-host disease. The donor variability shouldn’t be overlooked and therefore working collaboratively with the source provider to understand and control the incoming product should be encouraged. This will be the key to precision medicine where more and more treatments are funded based on outcome. It is imperative that a positive outcome is guaranteed for the therapy to be cost effective.
AUTOMATION OF THE DESIGN SPACE
Automation has brought many needed benefits to the industry. It aims to remove laborious processes that are heavily dependent on human interaction. It also aims to reduce wastage through process optimization and to reduce or eliminate in process errors. Ultimately, it ensures the processes are cost-effective and enables commercially viable manufacturing [5].
Automation may provide additional benefits in specific circumstances; sterile manufacturing processes are much easier to control, or many processes may look to adopt an information governance aspect that ensures integrity and traceability. The common goal in an automated process is to increase quality and reduce costs.
To help achieve this, QbD [10] can be employed to the automation process as a framework to guide the development phases. QbD is a mechanism in which you can test the impact of variability ensuring the automation effectively and reproducibly deals with variability so as the final product achieves the TQPP. We can characterize the input products, which enables us to assess the incoming variability and the process parameters or critical process attributes (the factors in the automation space that can be controlled); this could include centrifugation speeds and/or time, forces applied, cell densities or buffer exchange frequencies. These are all aspects that can have an impact on variability and the interconnecting relationship of these can be explored in the design space. Finally, the TQPP is the definition of what is being created, which allows for the critical quality attributes to be assessed in the design space, thus ensuring the TQPP is achieved.
The design space allows you to assess the impact of all known variables and understand how they affect the TQPP. Often, the process parameters are looked at in isolation (and rightly so in many circumstances) or the variability of the input products is corrected in the first step of the process in the design space, which may be normalized within a defined range. It is important to consider, however, that the input variability exists in the design space and may be a powerful tool that has a great impact on the TQPP.
Finally, once the variability is understood and the critical quality attribute’s and critical process attribute’s provide robust process predictability, the automation steps can be locked in into the control space. Figure 2
REJECTION
How can rejection be a solution to ensuring product specification? QbD is employed to ensure the product meets the specification. If it is done right there is no need to reject a product. Unfortunately, these are complex cell systems with variability at every level. Cells may be characterized, with a given variability, but may exhibit very different functional characteristics. This is certainly true from the cord setting. CD34+ positive cells are well characterized and in some settings correlate positively to transplant outcome, but intriguingly, we observe vastly different functionality within these units in their potency as assessed through colony forming unit assays. Characterization alone doesn’t imply function. What’s more, function doesn’t necessarily dictate fate. A high colony forming response in the functional assay may be indicative of short-term engraftment but could fall short of predicting long-term engraftment. The definable critical quality attributes will only go so far. A key principle of QbD is continual improvement. Judgements are made using mechanistic models and knowledge of these improves with time. The processes occupying the design space will need to be developed and redeveloped. Stem cell transplants from unrelated donors have been happening for over 40 years, over 200,000 hematopoietic progenitor cell products have been provided in the last 20 years alone, yet [11] even in this setting, we still get unpredictable negative results.
In the cord setting, we could control this more by identifying the earliest predictors that give the greatest possibility of success. In doing this we would only select cords from instrumental deliveries, as this gives the greatest number of CD34+ cells [8], and collect only from Caucasian donors as, in our experience, the volume of blood collected from Caucasian donors is higher. This more rigorous selection when combined with the robust automated processing and freezing systems that are employed would provide a product that is far more predictable and defined. Rejection of an unsuitable starting material would reduce the overall variability of the system, thus helping us to get closer to hitting the target, but we would miss the point as the bank of cords will not meet the needs of the patient pool. The nature of cord blood banking is to meet the unmet need which is largest in ethnic minorities – collecting solely from Caucasian donors would not serve the entire populous and as such, would be impractical as a pre-screening tool. The optimal collection may not be suitable for the patient, so we need to work within the variability pools of our patients. As a result, we accept greater variability and therefore accept rejection is a possible outcome.
It is also not cost effective to be pedantic: being too selective will rule out units that are less likely to meet specification, but a percentage still will. Using the ‘leaky funnel’ analogy (which implies that the more we have going into the top of the funnel – even if the structural integrity of said funnel is brought into question – we will still have more coming out of the bottom) the conundrum of efficient versus effective should always be evaluated, and accepting that there will be a rejection rate is important in weighing up its impact.
Learning from the registry and gained cord banking experience, the only reason we know so much about what has a positive impact on the TQPP and as a result, transplant success, is by collecting as much data as possible on the variability of donors, immunogenetics, the collection procedures, the processes, characterizations and functional assessment, to name a few. In order to control the variability, we first need to understand it. In the transplant setting we have hundreds of thousands of data points to draw on.
The development chain adds an additional dimension to consider. By examining different points in the development chain we can observe that there are different aspects to consider with regards to the selection, automation and rejection thresholds that could all be more flexible in the earlier stages. These parameters are all dictated by ethical, regulatory, scalability and translatability requirements, but more often than not, these are dictated by budget. Early stages of development could be earmarked for collating and understanding the impact of variability to better know whether it can be controlled or directed.
Is variability our enemy?
Quality by design aims to pre-emptively control variation by first measuring and understanding the variation that exists by using historical data, testing, and modeling to help forecast, analyze, and eliminate the deleterious effects of variation using standard statistical techniques [6].
Should we be in such a hurry to remove variability? We have touched on the inherent variability when using donors of the cells procured. These can be affected by genetics and any diseases or disease susceptibility, age [9], sex [12], whether the donors have had children [13] and the collection process [8]. All this variability is to be controlled during the manufacturing process to produce a defined TQPP, and to then go into a patient with as much if not more variability than the donors, due to their current disease state (Figure 3
In the registry setting, no amount of automation (with current technology) will control the HLA variability and matching donor characteristics to patient characteristics is vital. Again, this is an extreme example but there are lessons to the learnt based on the known variability beyond HLA we have seen from 40 years of selecting donors.
The aim of automation is to reduce the variability and ensure product consistency in a cost-effective manner. Given the limitations in present day knowledge, for the majority of cases, this could be an unrealistic expectation for automation alone and at best could be an opportunity to generate off the shelf products that can be selected and combined for patients whose variability is equally as big as that of the donors, if not greater.
CONCLUSION
The aim of the industry is to provide high quality products to treat complex disease indications in a cost-effective manner. To achieve this, some complex problems needs to be resolved before it can become available to patients on a mass scale.
To accelerate the development of novel cell therapy treatments and enable researchers to quickly and efficiently progress towards successful clinical trials. a multi-agency approach is needed between those that understand the donor challenges, those that understand the manufacturing capabilities and the clinicians selecting the appropriate treatment options for their patients.
At Anthony Nolan we help control the noise associated with donor variability, working to ensure the treatments meet specification by improving consistency, reproducibility and scalability through selection and provision of high-quality starting materials.
Today’s challenges and complexities are greater than we have seen before. The gains, however, are proving worthwhile as better treatment options are made available improving the patient survival and quality of life. Shirley Nolan started this journey to treat her son, who today would be a prime candidate for a gene therapy.
financial & competing interests disclosure
The authors are all employees of Anthony Nolan. Barring this they have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Cell and Gene therapy catapult Annual review (2018): https://ct.catapult.org.uk/our-impact/annual-reviews CrossRef
2. Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 UCB recipients with hematologic malignancies. Blood 2010; 115–24. CrossRef
3. Querol S, Gomez SG, Pagliuca A, Torrabadella M, Madrigal JA. Quality rather than quantity: the UCB bank dilemma. Bone Marrow Trans. 2010; 45: 970–8. CrossRef
4. Migliaccio AR, Adamson JW, Stevens CE, Dobrila NL, Carrier CM, Rubinstein P. Cell dose and speed of engraftment in placental/umbilical UCB transplantation: graft progenitor cell content is a better predictor than nuclear quantity. Blood 2000; 96: 2717–22. CrossRef
5. Wagner JE, Barker JN, Def TE et al. Transplantation of unrelated donor umbilical UCB in 102 patients with malignant and non malignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 2002; 100: 1611–8. CrossRef
6. Robinson J, Waller MJ, Fail SC, Marsh SGE. The IMGT/HLA and IPD databases. Nucleic Acids Res. 2015; 43: D423–431 CrossRef
7. Mancinelli F, Tamburini A et al. Optimizing umbilical cord blood collection: impact of obstetric factors versus quality of cord blood units. Transplantation Proc. 2006; 38: 1174–6 CrossRef
8. Lim FTH, Scherjon SA et al. Association of stress during delivery with increased numbers of nucleated cells and hematopoietic progenitor cells in umbilical cord blood. Am. J. Obs. Gynecol. 2000; 183(5): 1144–51 CrossRef
9. Shaw BE, Logan BR, Spellman SR et al. Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol. Blood Marrow Transplantation 2018; 24(5): 1049–56. CrossRef
10. Lipsitz YY, Timmins NE, Zandstra PW. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016; 34: 393–400. CrossRef
11. World Marrow Donor Association global trends report. 2017 annual report for WMDA global trends: https://www.wmda.info/wp-content/uploads/2018/06/20180531-GTR-Graphs-2017-Summary.pdf CrossRef
12. Gahrton G1, Iacobelli S et al. The impact of donor gender on outcome of allogeneic hematopoietic stem cell transplantation for multiple myeloma: reduced relapse risk in female to male transplants. Bone Marrow Transplant. 2005; 35(6): 609–17. CrossRef
13. Hirayama M1, Azuma E, Komada Y. Tolerogenic effect of non-inherited maternal antigens in hematopoietic stem cell transplantation. Front. Immunol. 2012; 3: 135. CrossRef
Affiliation
Daniel Gibson
Head of Innovation
& Commercialisation
Anthony Nolan
Christopher Leonforte
Processing and Quality Manager
Anthony Nolan Cell Therapy Centre
Prof. Alejandro Madrigal
Scientific Director
Anthony Nolan