Determination of physical viral vector titer in process development & QC for cell & gene therapy
Cell & Gene Therapy Insights 2022; 9(3), 399–411
DOI: 10.18609/cgti.2023.060
The rapid and efficient development, production, and release of viral vectors for cell and gene therapy depends on high-performance analytics that support process understanding and enable QC for release testing. Critical quality attributes (CQAs) include total physical viral vector titer and the ratio of full to empty capsids, which are the focus of this article. A range of analytical methods to measure these CQAs are being evaluated to support process analytical technology (PAT) and QC. While advanced methods such as analytical ultracentrifugation (AUC) and cryo-electron microscopy (cryo-EM) are in use or being evaluated to analyze the highly pure final product, the analysis of multiple samples of lower purity during process development requires another approach. A common method to determine the full:empty capsid ratio during process development involves combining genome data from quantitative PCR (qPCR) or, more recently, droplet digital PCR (ddPCR), with capsid titer data determined by enzyme-linked immunosorbent assay (ELISA). While ddPCR is an effective and precise assay method, ELISA has several drawbacks, including low throughput, high sample consumption, labor-intensive steps, and long turnaround times. The generation of viral vector capsid titer can be streamlined by using the Gyrolab system, which enables the miniaturization and automation of immunoassays to address these drawbacks.
Introduction
Since the first clinical trial conducted in 1990, major advances have been made in gene therapy, including the development of much-improved vectors. To date, most gene therapies utilize viral vectors, mainly adeno associated virus (AAV) and lentiviral (LV) vectors, to deliver the gene of interest. The major difference between LVs and AAVs is genome integration. LVs integrate their DNA into the host genome. This, together with the ability to express multiple genes means that LV vectors are frequently used to treat complex disease states such as congenital diseases, immune and metabolic disorders, and cancers. Genomic integration by LV prevents the dilution of genetic material over time due to cell division but poses a risk of oncogenesis. This problem is being addressed by third-generation, self-inactivating LV vectors that reduce the risk of insertional mutagenesis.
In contrast, genes delivered by AAVs become an episome, or circular piece of DNA that resides inside the nucleus. While a genome of ~4,7 kilobases (kb) limits the ability of AAV vectors to effectively package much more than ~5 kb, their extensive viral tropism means that AAV vectors are valuable for targeting gene therapies involving the heart, liver, and central nervous system.
Gene therapies represent over half of the 3726 gene, cell, and RNA therapies currently in development, with cancer and rare diseases as the main targets [1]American Society of Gene & Cell Therapy. Gene, Cell, & RNA Therapy Landscape report, Q4 2022. . The revolution in gene therapy development is putting significant pressure on bioprocess development and quality control (QC) to ensure that vectors can be quickly brought to the market.
This article focuses on one aspect of ensuring vector safety and efficacy – the determination of physical titer including empty, full, and partially-filled capsids, in process development and QC. We start by briefly looking at the regulatory landscape concerning vector analytics, particularly physical titer, and then summarize how different analytical methods fit the needs for determining titer in viral vector process development, production, and release testing. We conclude by illustrating how the measurement of capsid titer can be refined to ensure high data quality and productivity with an example involving the miniaturization and
automation of immunoassays.
The regulatory landscape
The US Food and Drug Administration (FDA) published revised regulatory guidelines for cell and gene therapy in January 2020 [2]Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, January 2020. . Overall, the FDA requires more detailed characterization and regulatory documentation for viral vector analytics regarding impurities, replication, titer, and infectivity.
The critical quality attributes (CQA) as mandated by the FDA’s chemistry, manufacturing, and control (CMC) guidelines for viral vector manufacturing include identity, strength/potency, purity, safety, and stability to ensure safety and efficacy. More specifically, the CQAs that should be monitored during development include “dosing units, genotypic or phenotypic variation, particle number and size, aggregation state, infectivity, specific activity (ratio of infectious to non-infectious particles or full to empty particles), biological activity or potency, and/or immunological activity)”.
Regarding product-related impurities, the Guidance states that, “For viral vectors, typical product-related impurities may include defective interfering particles, non-infectious particles, empty capsid particles, or replicating recombinant virus contaminants. These impurities should be measured and may be reported as a ratio, for example, full:empty particles or virus particles:infectious units”.
Similarly, the EMA guidelines on gene therapy products [13]US Food and Drug Administration. Toxicity Risks of Adeno-associated Virus (AAV) Vectors for Gene Therapy. Food and Drug Administration (FDA) & Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC) Meeting #70. September 2–3, 2021. state that, “The quantity of the drug substance should be established. For viral vectors, infectious titer should be quantified; the number of particles (infectious/non-infectious, empty/genome-containing) should also be determined. Particle to infectivity ratio should be included to define the content of the drug substance. For plasmids and other forms of nucleic acids, the quantity or concentration of nucleic acid should be established”.
Specific release criteria have also been indicated. For example, the proposed draft guidance for FDA consideration on testing AAV products for empty capsids [4]Dark Horse Consulting. Proposed DRAFT Guidance for FDA Consideration: Testing of Adeno-Associated Viral (AAV) Vector-Based Human Gene Therapy Products for Empty Capsids During Product Manufacture. Dark Horse Consulting Group. (May 15 2022). states that, “it is recommended that a maximum release criterion of ≤ 30% consisting of empty capsids be established for drug product. Accordingly, more than or equal to 70% of the product should consist of primarily full genome capsids”. However, Biophorum indicates that, “it is premature and impractical to set a minimal specification of less than 30% empty capsids to apply to all AAV-based gene therapies in development”. This organization suggests that the industry continues with a QbD risk-based strategy toward setting capsid specifications depending on the product [5]Biophorum. Control of empty full and partially filled capsids. Biophorum. (Accessed March 5 2023). .
Overview of titer analytics
The prospect of ensuring that a virus vector has the required purity for safe and effective use can be daunting. The key is to adopt a risk-based testing strategy in process development and final QC that minimizes the testing effort needed while meeting regulatory requirements and ensuring patient safety.
Process analytical technology (PAT) within the framework of Quality by Design (QbD) is becoming more widely applied to viral vector manufacturing. This has stimulated the development of advanced analytics that can quickly provide reliable data, including in-line testing, to improve process understanding to boost yields, improve vector safety, and lower costs. Rapid data generation for CQAs such as physical titer, including the full:empty ratio, is essential if techniques such as Design of Experiment (DoE) are to be effective. Analytical methods must therefore be rapid, accurate, and robust. Another important aspect is the ability to generate substantial amounts of data from limited amounts of precious sample, especially since regulatory demands are increasing the number of tests needed for batch release.
The testing strategy for viral vectors should include analytical methods with several important attributes that ensure the rapid and efficient generation of reliable data.
The need for speed to treat a select few
Cell and gene therapy can offer spectacular successes, including the treatment of patients suffering from genetic disorders previously thought to be incurable. Some therapies have been developed and approved for a relatively broad population, for example in the treatment of hemophilia A and B, and beta-thalassemia. Other therapies are only developed for a select few suffering from rare diseases and place particularly high demands on drug development. Patient populations are small, with personalized medicine sometimes being refined to truly individualized medicine, making treatments very expensive. Added to that, treatments are often fast-tracked from phase I for accelerated approval. Long assay times also add to the bottleneck in the development, production, and final QC of new products [6]Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity analysis for viral vector manufacturing. Is there enough? BioProcess Int. 2019; 17(11). [7]Transfiguracion J, Tran MY, Lanthier S et al. Rapid in-process monitoring of lentiviral vector particles by high-performance liquid chromatography. Mol. Ther. Meth. Clin. Dev. 2020; 18, 803–810., emphasizing the need for faster analytical approaches to characterize the therapeutic with regard to quality and titer [8]BioPharm. Gene Therapies Push Viral Vector Production – Bioprocess Development Forum. (May 21 2020). .
The demand for rapid turnaround times means that there is little time to validate new bioanalytical assay technologies, and rapid assay development and sample analysis are critical factors in reducing development times.
Increasing demands on data quality
As the number of clinical studies for AAV and LV-based gene therapies grows, the regulatory authorities are emphasizing the importance of vector titer assay reproducibility and the measurement of full:empty capsid ratios to facilitate dose comparison between clinical programs. For example, a recent workshop formulated a target of less than or equal to 15% precision for measurement of empty AAV capsids for early phase studies, which may require improvements in the reliability of analytical methods for viral vector titer [9]Christine Le Bec. Priorities for AAV vector analytics development. Cell Gene Ther. Insights 2020; 6(7), 871–875. [10]US Food and Drug Administration. Quantitation of AAV-based gene therapy products – 12/07/2018. FDA..
Getting more data from smaller sample volumes
Viral vector production is an expensive process that produces very little final product. For example, the product of a 200 L bioreactor can be concentrated down to 20 mL. Added to that, regulatory demands have increased the number of analyses required for characterization, putting an even higher premium on analytical techniques requiring less sample. It was estimated by one chemistry, manufacturing and control (CMC) specialist that almost half of the viral vector production batch may be consumed during QC bioanalysis steps [9]Christine Le Bec. Priorities for AAV vector analytics development. Cell Gene Ther. Insights 2020; 6(7), 871–875., which means that analytical methods that can process very small sample volumes are at a premium.
The need to measure capsid impurities
Capsid content characterization is a major challenge that puts a lot of pressure on analytics. Inefficiencies in viral vector production result in a fraction of viral particles that fail to package the vector DNA properly. This results in impurities that include empty capsids and capsids that contain nucleic acid sequences other than the desired vector genome.
Estimates of the distribution of AAV capsids during production are summarized in Table 1. Data from [11]Gimpel AL, Katsikis G, Sha S et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Mol. Ther. Meth. Clin. Dev. 2021; 20, 740–754.:
| Table 1. Distribution of AAV capsids during production. | ||
| Capsid type | Harvest (%) | Purified (%) |
| Full | < 30 | >70 |
| Partially filled | < 10 | < 1 |
| Empty | > 70 | < 30 |
Aggregates can also be present at different levels (small less than 2% and large less than 1 ppm).
Taking AAV as an example, empty capsids can have several negative effects that threaten safety and efficacy [13]US Food and Drug Administration. Toxicity Risks of Adeno-associated Virus (AAV) Vectors for Gene Therapy. Food and Drug Administration (FDA) & Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC) Meeting #70. September 2–3, 2021. :
- Increasing the overall antigenic load that may exacerbate innate and adaptive immune responses;
- Contributing to the peptides presented by major histocompatibility complex (MHC) molecules, with consequent recognition and clearance of transduced cells by capsid-specific cytotoxic T cells;
- Functioning as a pathogen-associated molecular pattern (PAMP) that can be recognized by toll-like receptor (TLR) 2, resulting in the induction of innate immune responses;
- Competing with full capsids for receptor binding, which could necessitate a dose increase.
While the presence of empty capsids can have benefits in certain situations, for example as decoys for anti-AAV antibodies to enhance gene transfer, minimizing the level of empty capsids generally improves safety, especially when high vector doses are administered in clinical studies [13]US Food and Drug Administration. Toxicity Risks of Adeno-associated Virus (AAV) Vectors for Gene Therapy. Food and Drug Administration (FDA) & Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC) Meeting #70. September 2–3, 2021. . Removing AAV empty capsids during manufacture is a real challenge, especially during scale-up, which means that reducing the load by optimizing upstream and downstream processes is critical.
A recent draft guidance regarding AAV testing for FDA consideration [6]Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity analysis for viral vector manufacturing. Is there enough? BioProcess Int. 2019; 17(11). proposed identifying the following product impurities:
- Empty capsids;
- Non-infectious AAV;
- Aggregated AAV;
- Replication-competent AAV;
- Encapsidated host-cell DNA;
- Encapsidated helper plasmid DNA;
- Encapsidated partial genome*;
- Encapsidated mutated* or methylated genome;
- Capsid post-translational modifications (PTMs)*.
- * not included in prior FDA recommendations
Analytics for capsid titer CQAs
Several reviews have summarized the wide range of analytical methods available for determining capsid titer and genome titer [14]Hutanu A, Boelsterli D, Schmidli C, et al. Stronger together: Analytical techniques for recombinant adeno associated virus. Electrophoresis. 2022; 43(9–10), 1107–1117. [15]Shmidt AA, Egorova TV. PCR-Based Analytical Methods for Quantification and Quality Control of Recombinant Adeno-Associated Viral Vector Preparations. Pharmaceuticals (Basel). 2021; 15(1), 23. [16]Werle AK, Powers TW, Zobel JF et al. Comparison of analytical techniques to quantitate the capsid content of adeno-associated viral vectors. Mol. Ther. Meth. Clin. Dev. 2021; 23, 254–262. [17]Sylvestre J, Conti-Permanne P. Emerging technologies & companies in cell & gene therapy manufacturing. Cell Gene Ther. Insights 2022; 8(11), 1601–1649.. In a draft guidance for FDA consideration, the consulting firm Dark Horse narrowed the field by proposing the methods shown in Table 2.
| Table 2. Analytical methods for determining capsid titer and genome titer, as proposed by Dark Horse. | |||||
| Method | Throughput | Ease of use | Material used | Partial genomes | Accuracy/precision |
| Charge detection mass spectrometry (CDMS) | + But requires buffer exchange | - Specialized equipment | ++ | ++ | ++ |
| ELISA + ddPCR | + But d(d)PCR requires sample treatment | ++ Commonly used | ++ | - | - |
| Size exclusion chromatography with multi-angle light scattering (SEC-MALS) | ++ | + Relatively common equipment | + | - | + |
| Transmission electron microscopy (TEM) | - Sample staining, low throughput | - Specialized equipment | ++ | - | - |
| Analytical ultracentrifugation (AUC) | - | - Specialized equipment | + | ++ | ++ |
| Based on guidance proposal from Dark Horse [4]. | |||||
There are additional aspects of these techniques that should be pointed out:
- Charge detection mass spectrometry (CDMS) measures the charge and mass-to-charge ratio of individual ions and can be used to resolve empty, partially filled, and full capsids with a repeatability of less than 2% CV and a turnaround time of
2 h. But the method is less mature than, for example, analytical ultracentrifugation (AUC) [11]. - Transmission electron microscopy (TEM) has shown problems with poor agreement with orthogonal methods, low throughput, and long turnaround time [11].
- AUC is highly repeatable (2% CV) and can be used to resolve partially filled, empty, and full capsids. But this method consumes a lot of material (400–500 µL sample) and has a throughput of only seven samples in 6 h, making it more suitable as an orthogonal method to validate more rapid methods [11].
- Methods to measure partially filled capsids include AUC and CDMS shown in Table 2, and also Cryo-EM [12].
A common approach used today for measuring the full:empty ratio therefore involves measuring the genome content and capsid content separately and then using the quotient to determine the % of full:empty capsids. Quantitative polymerase chain reaction (qPCR) or digital droplet PCR (ddPCR), which is replacing qPCR, are widely used methods to quantify genome titer due to their simplicity, specificity, and robustness. They are based on fluorescence detection of specific DNA sequences during amplification (qPCR) or after amplification (ddPCR) in a thermocycler. Both require sample preparation to remove non-encapsidated DNA and denature capsid proteins to expose the encapsidated DNA. qPCR is the standard procedure for determining genome titer of rAAV reference standard material (RSM) but suffers from low precision, with repeatability as low as >30 %CV and reproducibility of 70–100%. In contrast, ddPCR, which does not require a standard curve and measures the endpoint of PCR cycles, has a repeatability of 2–20 %CV [11]Gimpel AL, Katsikis G, Sha S et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Mol. Ther. Meth. Clin. Dev. 2021; 20, 740–754..
ELISA is the most common method for determining capsid titer and has a high specificity for intact capsids and is relatively robust to matrix effects. This method can deliver acceptable performance when used to determine AAV capsid titer, with a repeatability of 10–15 %CV and reproducibility of around 40 %CV [11]Gimpel AL, Katsikis G, Sha S et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Mol. Ther. Meth. Clin. Dev. 2021; 20, 740–754.. This traditional plate-based method suffers from several disadvantages, however, including low throughput (10 samples per 96-well plate), high sample consumption, and requires labor-intensive steps together with turnaround times of several hours.
Translational insight
Miniaturization & automation boost immunoassay performance
The combination of data from qPCR or ddPCR and ELISA is often used to generate data on full:empty capsid ratios, but data quality can be compromised by the accumulated error resulting from combining results from two analytical methods. The shift from qPCR to ddPCR can improve the repeatability of genome measurements but there remains a need for the efficient and rapid determination of physical capsid titer with high accuracy and repeatability. ELISA is a well-established method to determine capsid titer but has relatively low throughput, narrow analytical range, requires many manual steps, and consumes relatively large volumes of sample. The question is, how the immunoassay-based determination of capsid titer can be improved to support the rapid generation of high-quality data?
The key factors in choosing an immunoassay platform are summarized in Table 3.
| Table 3. The key factors in choosing an immunoassay platform. | |
| Key factors | Benefit |
| High precision and accuracy | Confidence in decisions |
| Broad analytical range | Reduces need for dilutions and repeats |
| Robustness | Reliable and repeatable data |
| Matrix tolerance | Enable the analysis of complex samples with low minimum required dilution (MRD), which improves functional sensitivity. |
| Rapid data generation | Meet tough timelines |
| High throughput | Efficiently handle large sample sizes in development |
| Flexible open platform | Run multiple assays in parallel to save time Enable the development of novel assays |
| Automation | Free up scientist’s time for other critical tasks Reduces risk of error |
| Low sample- and reagent consumption | Ensure maximum data generation with the minimum of precious samples and reagents |
| Easily sanitized | Meet biosafety requirements when working with viral vectors |
| Readily validated and 21 CFR Part 11 compliant software | Meet the demands of regulatory guidelines |
Gyrolab system has been developed by Gyros Protein Technologies to address the requirements listed in Table 3 and is now well established in the biotech and pharmaceutical industry for a wide range of applications, including vector quantitation and characterization, host cell protein impurity measurement, and monitoring in vitro potency. Kits are available to determine titers of AAV serotypes 1–10 and the p24 antigen of LV. The principle of a Gyrolab assay is shown in Figure 1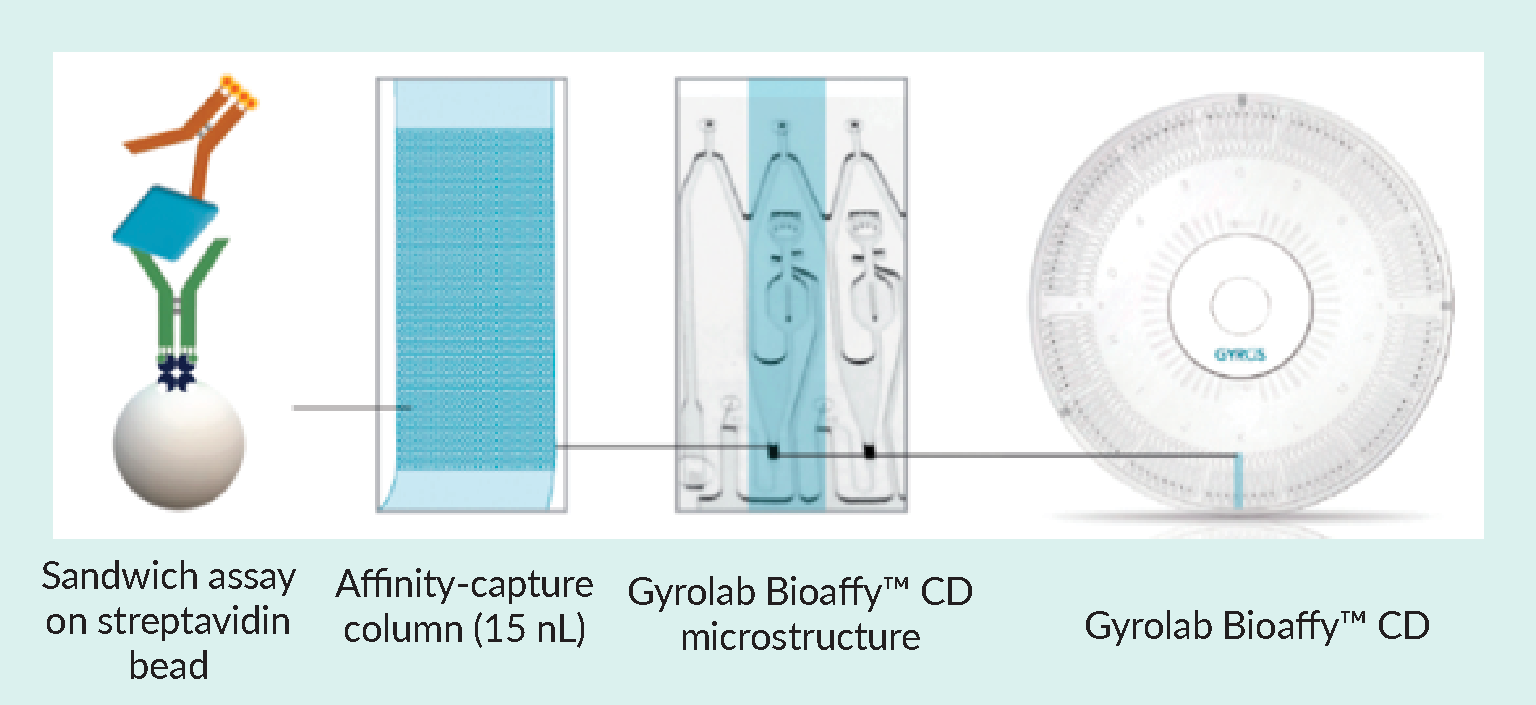 Gyrolab® BioaffyTM CD-based microfluidic immunoassay design utilizing a 15 nL affinity capture column, streptavidin beads, and microstructures in a circular array for precise, automated liquid movements using centrifugal force.Parallel processing of Gyrolab CD-based immunoassays on streptavidin beads within the affinity capture column uses centrifugal force and capillary action to precisely control the flow of reagents and samples over the column. On-column laser-induced fluorescence results are read automatically, and results are ready to analyze at the end of the run. The short contact times minimize matrix interference and dramatically shorten assay times..
Gyrolab® BioaffyTM CD-based microfluidic immunoassay design utilizing a 15 nL affinity capture column, streptavidin beads, and microstructures in a circular array for precise, automated liquid movements using centrifugal force.Parallel processing of Gyrolab CD-based immunoassays on streptavidin beads within the affinity capture column uses centrifugal force and capillary action to precisely control the flow of reagents and samples over the column. On-column laser-induced fluorescence results are read automatically, and results are ready to analyze at the end of the run. The short contact times minimize matrix interference and dramatically shorten assay times..
The automation and miniaturization of the flow-through assays afforded by Gyrolab technology results in several benefits over plate-based ELISA (Table 4 & Figure 2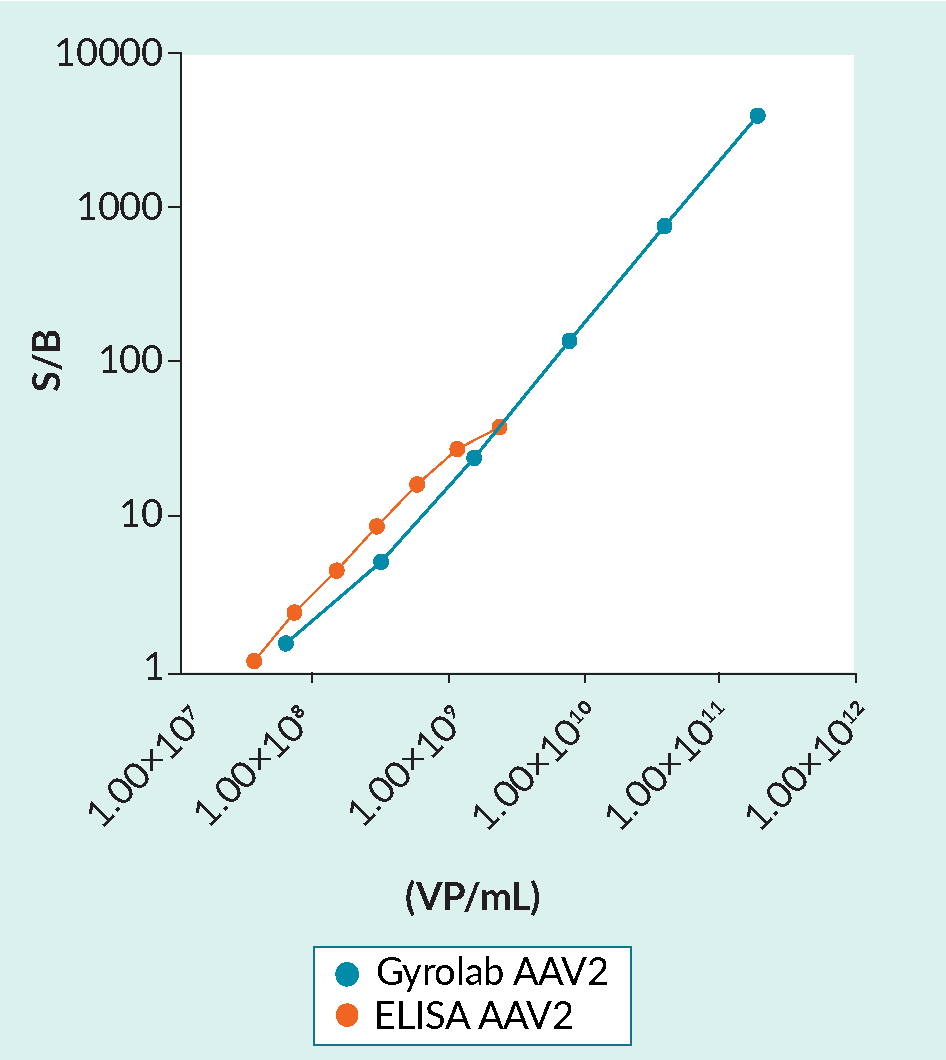 Gyrolab AAVX capsid titer immunoassay performance versus ELISA.The broad dynamic range of Gyrolab AAV immunoassays reduces the need to dilute or re-run samples. The 2-log increase in dynamic range is especially useful in high-titer AAV batch production. The Gyrolab AAV2 immunoassay was performed using Gyrolab AAVX Titer Kit. ELISA was performed according to the kit instructions (PROGEN). AAV2 standards (Sirion Biotech GmbH) were measured in duplicate after dilution in steps of 1:5 from 2.0×1011 VP/mL or in steps of 1:2 from 2.4×109 (ELISA). (S/B, signal/background; VP/mL, viral particles per mL).).
Gyrolab AAVX capsid titer immunoassay performance versus ELISA.The broad dynamic range of Gyrolab AAV immunoassays reduces the need to dilute or re-run samples. The 2-log increase in dynamic range is especially useful in high-titer AAV batch production. The Gyrolab AAV2 immunoassay was performed using Gyrolab AAVX Titer Kit. ELISA was performed according to the kit instructions (PROGEN). AAV2 standards (Sirion Biotech GmbH) were measured in duplicate after dilution in steps of 1:5 from 2.0×1011 VP/mL or in steps of 1:2 from 2.4×109 (ELISA). (S/B, signal/background; VP/mL, viral particles per mL).).
| Table 4. Performance of Gyrolab AAVX capsid titer immunoassay exceeds ELISA performance and suitability for bioprocess development. | ||
| ELISA | Gyrolab system | |
| Sample volume required | 100–200 μL | 8 μL |
| Number of hands-on steps | 5 | 1 |
| Total assay time | 4 h | 1 h |
| Dynamic range | 1–2 logs | > 3 logs |
When compared to ELISA kits, Gyrolab microfluidic immunoassays greatly reduce the sample volumes, hands-on time required, and overall assay time, while extending the assay dynamic range. These dramatic improvements in assay performance and sample consumption meet the demands for vector titer bioanalysis required by the compressed production timelines and limitations on batch yields.
The high quality of data generated using Gyrolab assays can be seen in Figure 2, Figure 3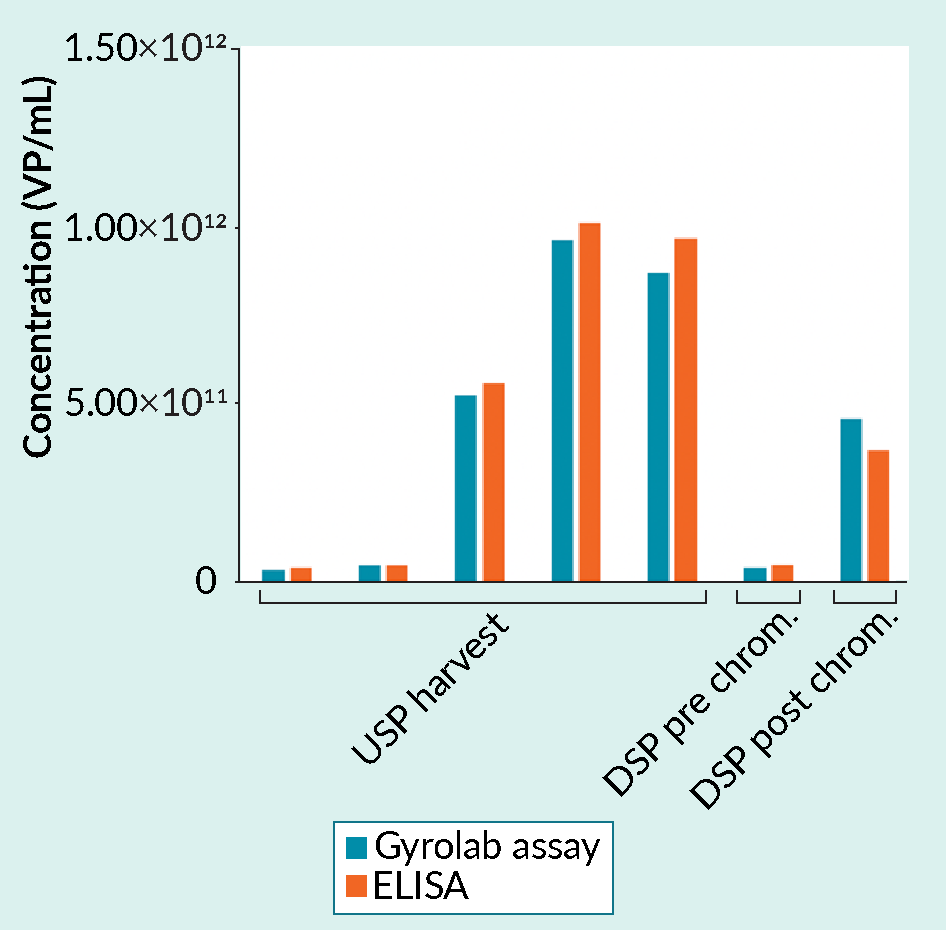 Analyzing samples from upstream and downstream processing: Gyrolab assay versus ELISA.Gyrolab AAV9 Titer Kit and a manual capsid ELISA gave comparable results for a range of samples. The data was supplied by a CRO providing analytical services for cell and gene therapy customers. USP, upstream process; DSP, downstream process. and Figure 4
Analyzing samples from upstream and downstream processing: Gyrolab assay versus ELISA.Gyrolab AAV9 Titer Kit and a manual capsid ELISA gave comparable results for a range of samples. The data was supplied by a CRO providing analytical services for cell and gene therapy customers. USP, upstream process; DSP, downstream process. and Figure 4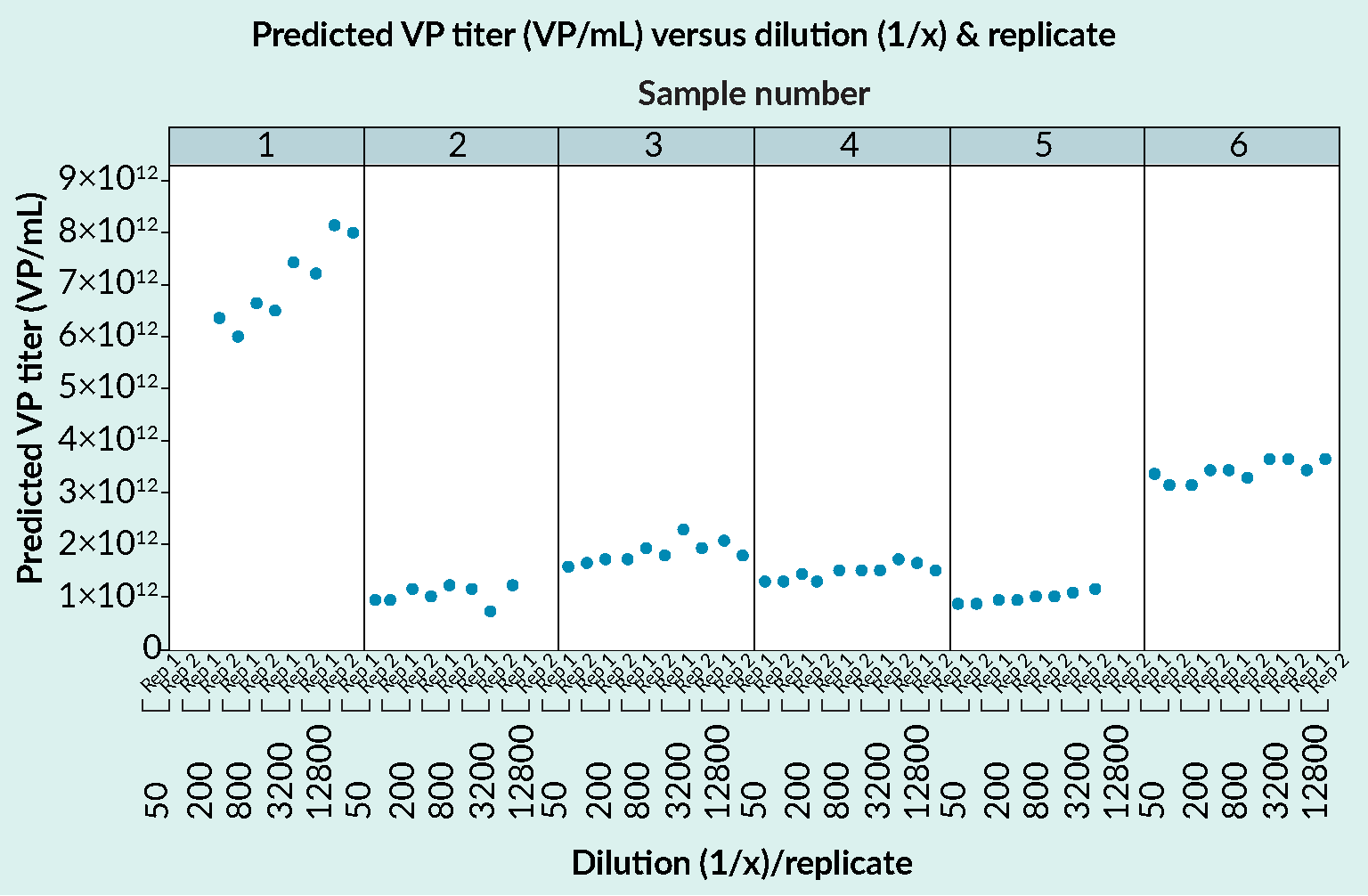 Gyrolab AAVX Titer Kit results in the determination of capsid titer for AAV5.Gyrolab AAVX Titer Kit shows good dilutional linearity with comparable determinations for viral particle (VP) titer whether the sample was diluted 1:50 or 1:12800. Data is for serotype AAV5. and Tables 5 & 6.
Gyrolab AAVX Titer Kit results in the determination of capsid titer for AAV5.Gyrolab AAVX Titer Kit shows good dilutional linearity with comparable determinations for viral particle (VP) titer whether the sample was diluted 1:50 or 1:12800. Data is for serotype AAV5. and Tables 5 & 6.
| Table 5. Gyrolab AAVX Titer Kit: representative accuracy and precision data for seven QC samples for the determination of the working range when determining AAV2 capsid titer. | ||||||
| Sample | Expected conc (VP/mL) | Average measure conc. (VP/mL) | Intra-run CV (%) | Inter-run CV (%) | Average accuracy (%) | Average TE (%) |
| ULOQ 1 | 1.87×1011 | 1.86×1011 | 1.67 | 2.8 | 99.3 | 0.93–8.16 |
| ULOQ 2 | 1.26×1011 | 1.22×1011 | 2.52 | 5.3 | 96.6 | 2.47–12.24 |
| MQC | 9.16×109 | 9.27×109 | 4.03 | 4.1 | 101.2 | 4.80–12.92 |
| LQC | 4.65×108 | 4.56×108 | 2.49 | 2.3 | 98.0 | 2.30–9.79 |
| LLOQ 1 | 1.76×108 | 1.74×108 | 7.91 | 4.5 | 98.7 | 6.55–17.86 |
| LLOQ 2 | 1.22×108 | 1.20×108 | 9.15 | 10.9 | 98.3 | 13.12–27.22 |
| LLOQ 3 | 9.85×107 | 8.94×107 | 1.18 | 5.5 | 90.8 | 9.30–39.94 |
| Table 6. Gyrolab p24 Kit: Intra- and inter-run precision for the standards used to prepare the standard curve. | ||||
| Expected conc (ng/mL) | Average measured conc (ng/mL) | Intra-run CV (%)1 | Inter-run CV (%)2 | |
| Blank | ||||
| Standard 13 | 1250 | 1250 | 3.6 | 3.1 |
| Standard 2 | 250 | 251 | 2.3 | 1.9 |
| Standard | 50 | 50 | 2.8 | 2.7 |
| Standard 4 | 10 | 10 | 2.9 | 2.8 |
| Standard 5 | 2 | 2 | 1.7 | 2.1 |
| Standard 6 | 0.4 | 0.4 | 2.0 | 1.8 |
| Standard 7 | 0.08 | 0.08 | 5.0 | 5.3 |
| 1Intra-run CV (%) = standard deviation of response divided by mean response from one run performed in duplicates. 2Inter-run CV (%) = standard deviation of means from six runs performed in duplicates divided by mean response for the six runs. 3Purified recombinant p24 standards diluted in assay buffer. | ||||
In the context of ICHQ2(R1) [18]FDA Q2(R1) Validation of Analytical Procedures: Text and Methodology Guidance for Industry. , these data summarize repeatability (intra-assay precision), intermediate precision (inter-run precision), linearity, and range.
Gyrolab AAVX Titer Kit has working ranges of 1×108–1×1011 for serotypes AAV1 – AAV7 and AAVrh10, and 1×109–1×1012 for AAV8. A separate kit is available to measure AAV9 titer. Table 5 shows data for standard curves and QC samples over the working range of the Gyrolab AAVX Titer Kit when used to measure AAV2 titer. Samples were run in duplicate in six runs on four instruments by three operators. Six duplicate runs were performed on four different instruments, or N=12 per standard concentration. The intra- and inter-run precision was well under 10% (1.7–5.3%), demonstrating an extremely robust assay.
Table 6 shows similar accuracy and precision data for the quantification of LV capsid titer by determining p24 antigen using Gyrolab 24 Kit.
Further support for the suitability of Gyrolab system in the determination of capsid titer is shown in Figures 3 and 4. Gyrolab assays deliver comparable data to ELISA when used to analyze a range of samples from upstream and downstream processing (Figure 3) and the assays show high dilutional linearity (Figure 4).
Discussion
Rapid advances in cell and gene therapy include the development and evaluation of a wide range of analytical techniques to determine the CQAs needed to guide process development and support QC and final release testing. Advanced methods such as AUC and cryo-EM for final release testing are being evaluated to generate data on empty, full, and partially filled capsids in one analysis but require complex instrumentation. Immunoassays, on the other hand, are based on readily available instrumentation and can generate data relatively quickly to support process development in particular.
The determination of CQAs such as capsid titer, including full:empty ratios, for process development and final QC, relies on the availability of analytics that can quickly deliver high-quality reliable data with a minimum of effort and sample. Plate-based immunoassays (ELISA) are commonly used to measure physical virus/capsid titer in process development and QC. Measuring the CQA, empty:full ratio, means combining the capsid data with genome data generated using PCR-based methods, which results in accumulated error and necessitates the development of individual methods with high precision. The need for high precision, for example, was noted in an interview with Christine Le Bec in Cell & Gene Therapy Insights, with a target for precision of less than or equal to 15% CV for the measurement of empty AAV capsids being recommended for early phase studies [9]Christine Le Bec. Priorities for AAV vector analytics development. Cell Gene Ther. Insights 2020; 6(7), 871–875..
In the case of genome determinations, the need for increased precision has resulted in a shift from using qPCR to ddPCR to improve data quality. On the other hand, the generation of capsid data using plate-based immunoassays has several disadvantages, including the need for large sample volumes, relatively laborious and time-consuming workflows, and limited dynamic range. Gyrolab system has been developed to address these problems and illustrates how technology development can support cell and gene therapy in a similar way to the shift from qPCR to ddPCR for genome determinations. Gyrolab assays can quickly deliver data with a precision of better than 10 %CV, which matches the performance of ddPCR (repeatability 2%–10% CV [11]Gimpel AL, Katsikis G, Sha S et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Mol. Ther. Meth. Clin. Dev. 2021; 20, 740–754.) to increase the precision of not only total capsid determinations but also full:empty ratios. The microfluidic design, flow-through affinity column, and automation all contribute to the high reproducibility both within runs and between runs.
Gyrolab system and associated kits can also be used to measure other impurities, such as host cell proteins (HCPs), endonuclease, and transferrin. The automation increases throughput and reduces risk of error, and the system is readily validated and is supported by 21 CFR Part 11 compliant software.
Conclusion
The application of Gyrolab technology represents just one example of the search for analytical methods with short turnaround times, high throughput, and simple sample preparation that deliver reliable data for a wide range of CQAs in vector development and production. These efforts will help address bottlenecks in vector production and support process understanding, with the goal of matching advances made in the production of other complex pharmaceuticals and the timely release of safe and efficacious cell and gene therapeutics.
Biographies
Daniel Forsström is R&D Application and Custom Service Manager at Gyros Protein Technologies. He leads the RnD Application and Custom service teams for Gyros Protein Technologies and is responsible for developing new assays, new products and custom assay solutions for the Gyrolab platform. He has obtained vast experience developing analytical assays for high throughput technologies used in preclinical and clinical drug development after approximately 15 years in various positions at different biotech companies.
John Chappell has approximately 25 years of experience in the Contract Research industry supporting both preclinical and clinical drug development. He has specialized in supporting biological compounds from an analytical perspective e.g. Pharmacokinetic, Immunogenicity and Biomarker analysis. He is particularly interested in validation requirements and ensuring that data generated will be acceptable to the regulatory authorities. He has spoken at many international conferences on various topics including Oligonucleotide analysis, Biomarker Analysis, Immunogenicity and the analytical support of Biosimilar programs. He now leads the Application Support and Service teams for Gyros Protein Technologies where he is responsible for customer service and technical support in Europe and the Asia Pacific regions. John has been a user of the Gyrolab® system for over 10 years so will use this experience to help customers. He is a Fellow of the Royal Society of Chemistry and was involved in the American Association of Pharmaceutical Scientists (AAPS) Biosimilar Committee that has prepared papers on Pharmacokinetic and anti-drug antibody assays.
Affiliations
Daniel Forsström
Gyros Protein Technologies
John Chappell
Gyros Protein Technologies
References
1. American Society of Gene & Cell Therapy. Gene, Cell, & RNA Therapy Landscape report, Q4 2022. Crossref
2. Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, January 2020. Crossref
3. European Medicines Agency. Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products. EMA/CAT/80183/2014. (March 22 2018). Crossref
4. Dark Horse Consulting. Proposed DRAFT Guidance for FDA Consideration: Testing of Adeno-Associated Viral (AAV) Vector-Based Human Gene Therapy Products for Empty Capsids During Product Manufacture. Dark Horse Consulting Group. (May 15 2022). Crossref
5. Biophorum. Control of empty full and partially filled capsids. Biophorum. (Accessed March 5 2023). Crossref
6. Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity analysis for viral vector manufacturing. Is there enough? BioProcess Int. 2019; 17(11). Crossref
7. Transfiguracion J, Tran MY, Lanthier S et al. Rapid in-process monitoring of lentiviral vector particles by high-performance liquid chromatography. Mol. Ther. Meth. Clin. Dev. 2020; 18, 803–810. Crossref
8. BioPharm. Gene Therapies Push Viral Vector Production – Bioprocess Development Forum. (May 21 2020). Crossref
9. Christine Le Bec. Priorities for AAV vector analytics development. Cell Gene Ther. Insights 2020; 6(7), 871–875. Crossref
10. US Food and Drug Administration. Quantitation of AAV-based gene therapy products – 12/07/2018. FDA. Crossref
11. Gimpel AL, Katsikis G, Sha S et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Mol. Ther. Meth. Clin. Dev. 2021; 20, 740–754. Crossref
12. Subramanian S, Maurer AC, Bator CM et al. Filling Adeno-Associated Virus Capsids: Estimating Success by Cryo-Electron Microscopy. Hum. Gene Ther. 2019; 30(12), 1449–1460. Crossref
13. US Food and Drug Administration. Toxicity Risks of Adeno-associated Virus (AAV) Vectors for Gene Therapy. Food and Drug Administration (FDA) & Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC) Meeting #70. September 2–3, 2021. Crossref
14. Hutanu A, Boelsterli D, Schmidli C, et al. Stronger together: Analytical techniques for recombinant adeno associated virus. Electrophoresis. 2022; 43(9–10), 1107–1117. Crossref
15. Shmidt AA, Egorova TV. PCR-Based Analytical Methods for Quantification and Quality Control of Recombinant Adeno-Associated Viral Vector Preparations. Pharmaceuticals (Basel). 2021; 15(1), 23. Crossref
16. Werle AK, Powers TW, Zobel JF et al. Comparison of analytical techniques to quantitate the capsid content of adeno-associated viral vectors. Mol. Ther. Meth. Clin. Dev. 2021; 23, 254–262. Crossref
17. Sylvestre J, Conti-Permanne P. Emerging technologies & companies in cell & gene therapy manufacturing. Cell Gene Ther. Insights 2022; 8(11), 1601–1649. Crossref
18. FDA Q2(R1) Validation of Analytical Procedures: Text and Methodology Guidance for Industry. Crossref
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors are employees of Gyros Protein Technologies.
Funding declaration: The authors received financial support for the research, authorship and/or publication of this article from Gyros Protein Technologies.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2023 Gyros Protein Technologies. Published by Cell and Gene Therapy Insights Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Mar 9 2023; Revised manuscript received: Apr 26 2023; Publication date: May 12 2023.