Tissue Manufacturing by Bioprinting: Challenges & Opportunities
Cell Gene Therapy Insights 2018; 4(8), 781-790.
10.18609/cgti.2018.081
Despite substantial investments to meet clinical and commercial expectations, and while scientific achievements at the preclinical research stage have sometimes been impressive, scaffold-based tissue engineering approaches are struggling to find the way to therapeutic and industrial success. The main challenges for the manufacture of tissue-engineered Advance Therapy Medicinal Products (ATMPs) concern the improvement of the standardization of manufacturing processes, tissue functionality and cost–effectiveness and profitability of related treatments. Based on our experience in the field of bioprinting, we discuss how this technology – thanks to its characteristics resulting from the convergence of automation, biology and digital technology – should make it possible to overcome current tissue manufacturing bottlenecks and also provide new opportunities.
Submitted for Peer Review: Aug 18 2018 Published: Nov 6 2018
Introduction
The need for tissue and organ transplantation has increased dramatically worldwide over the past decade, predominantly due to the increase in life expectancy, the incidence of vital organ dysfunction and degenerative diseases, and the need to address the consequences of tumor removal. Because of this organ shortage, in the European Union alone, more than 63,000 patients are waiting for an organ transplant (kidney, liver, heart, cornea, etc.), and 6 new patients are added every hour to the waiting lists [1]. By contrast, only about 33,000 donors were identified in 2016 [2]. While some countries are implementing policies to increase organ donation, many patients waiting for a transplant will not receive it in time. Patients and clinicians are therefore waiting for tissue and organ substitutes that must be thoroughly characterized and safe, ideally patient specific and potentially ‘off-the-shelf’. In that context, the global tissue engineering (TE) market is expected to reach $11.5 billion by 2022 [3].
Research scientists and engineers working in the field of TE apply the principles of biology and engineering to develop biological substitutes that restore, maintain or improve tissue function [4]. TE approaches have traditionally relied on the use of biocompatible materials, shaped to form a 3D scaffold on which living human cells are seeded before maturation in a bioreactor. As the cells multiply, they colonize the scaffold and synthesize an extracellular matrix (ECM) to create a 3D tissue. Despite substantial investments to meet clinical and commercial expectations, and while scientific achievements at the preclinical research stage have sometimes been impressive, these traditional TE approaches are struggling to find the way to therapeutic and industrial success [5]. Four tissue-engineered products (TEP) belonging to the category of Advanced Therapy Medicinal Products (ATMP) have indeed obtained to date their European marketing authorization by the European Medicines Agency (EMA), and even in these cases, the therapeutic benefit did not meet medical expectations and marketing was not profitable [6]. This is illustrated by the withdrawal from the European market of two of these ATMPs which had previously demonstrated sufficient safety and efficiency: MACI®(2014), Chondrocelect® (2016); only Holoclar® and Spherox products are now commercially available.
To meet medical and commercial expectations, TEP manufacturing presents unique challenges that need to be addressed. These challenges concern the improvement of:
- Standardization of tissue manufacturing processes, in particular cell seeding, which are still mainly carried out manually, in a craftsman-like manner, in the absence of supervision systems and through many open stages. Such standardization will facilitate compliance with the various regulatory requirements (and in particular Good Laboratory Practice [GLP] and Good Manufacturing Practice [GMP]), which frame preclinical development and clinical batch production, with the aim of ensuring:
- Tissue safety
- Manufacturing stability, which guarantees reproducibility of process performance and product quality
- Comparability of manu-facturing results obtained at different sites, by different operators and between the preclinical and clinical phases
- Industrial scale-up and scale-out
- The functionality of the engineered tissues so that the associated therapies are more effective than alternative treatments by medical devices, drug treatments or self-transplant;
- The cost–effectiveness and profitability of TEP to make them accessible to patients and bearable for insurance systems.
To meet these challenges, bioprinting approaches have been developed. These are based on “the use of computer-aided transfer processes for patterning and assembling living and non-living materials with a prescribed 2D or 3D organization in order to produce bio-engineered structures serving in regenerative medicine, pharmacology and basic cell biology studies” [7–9]. Despite similar principles, it should be noted that bioprinting differs from 3D printing of prosthetic implants since the nature of the material deposited is living and not inert, and also by the technologies used. Bioprinting has gained a lot of attention during recent years, namely due to the significant progress made towards the manufacture of implantable bioprinted tissues. These advances are namely: fabrication of vascularized thick tissues [10]; preclinical studies of bioprinted cardiac patches [11] or trachea [12] in animals; as well as industrial production and marketing bioprinted skin and liver as physiological models for in vitro applications [13,14]. As for technology, several bioprinting techniques have been developed so far: bioprinting using thermal and piezoelectric inkjet printers, micro-valve bioprinting, bio-extrusion and laser assisted bioprinting. For more information on all these techniques, readers can refer to references [15,16].
Briefly, inkjet bioprinting consists in projecting micro-droplets of a liquid containing cells onto a substrate. The projection is caused by a thermal or piezoelectric process. Thermal inkjet printing relies on the transient activation of a thermal resistance that produces a vapor bubble that propels a droplet through an orifice from 30 to 200 µm in diameter. Piezoelectric inkjet printers use an electrical pulse that generates a shape change of a piezoelectric crystal that contracts the ink reservoir. The relaxation of the crystal causes the ejection of the drop. The advantage of this technique is its low cost and speed, whether this is in terms of preparation time or printing speed. However, inkjet is limited by the viscosity of the bioink used and its inability to operate at high cell densities where concentrations of only few cell/mL have to be maintained to prevent clogging of the printheads, as well as the cellular mortality that results from shear stress on the cells when passing through the orifice.
Micro-valve bioprinting is similar to inkjet technologies, but differs in that jet formation occurs by pressurizing the ink and quickly opening a solenoid valve. With the same constraints as inkjet bioprinting, this technology brings the capacity to print more viscous solutions and tends to suffer far less from nozzle clogging even with higher cellular concentrations.
Bioextrusion consists in mechanically displacing biological elements placed in a micro-syringe through a nozzle or needle a few hundred micrometers in diameter. The advantage of this technology lies in its low cost and ease of implementation. However, it suffers from limitations associated with coarse resolution and significant cell mortality due to the shear imposed on cells as they pass through the nozzle.
Laser-assisted bioprinting is finally the main orifice-free technology developed to date. It is based on the use of a pulsed laser source that induces the transfer of bioink microdroplets from a target – made of a glass slide covered with a thin layer of bioink – to a receiving substrate placed a few hundred micrometers away. The main advantage of laser-assisted bioprinting is that it allows the deposition of cells with very high resolution, up to the single cell level. The absence of an orifice also ensures cell viability reaching values of greater than 95%. However, this technology still suffers from a low throughput.
Whatever the bioprinting technology used, the manufacture of biological human tissues can be divided into a sequence of five technological steps:
- Computer Aided Design (CAD) of a digital blueprint that defines tissue architecture, what means the spatial location of all tissue components (cell suspensions, cell aggregates, biomaterials such as collagen or growth factors);
- Programming the printing sequence and bioink printing parameters which leads to defining the printer’s physical parameters and the printhead trajectories;
- Bioink preparation and formu-lating, and cartridge filling;
- Layer-by-layer deposition of bioinks using automated (possibly robotic) systems integrated into the bioprinters;
- Maturation of bioprinted tissue into bioreactors.
Upstream of this sequence, tomographic reconstructions can be performed to help tissue design (e.g., through microscopic, histological or medical imaging) and the cells are prepared by conventional extraction, isolation, amplification and differentiation steps. Downstream, the bioprinted tissues could be conditioned/packaged before being sent to the medical centre and implanted into patients (Figure 1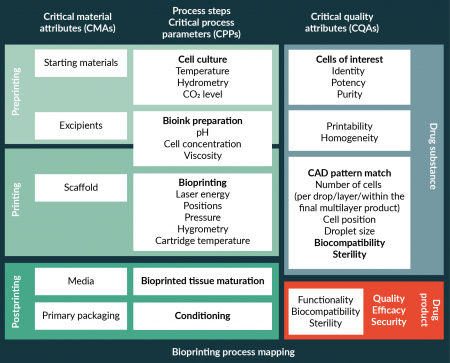
Bioprinting requirements for manufacturing & commercializing TEPs
Poietis experience in the field of bioprinting relies in particular in the development of several biological tissues (such as skin, but also complex tissues like hair follicle in the framework of a partnership with L’Oréal) as well as in the industrialization of the Poieskin® full thickness skin model. Marketed for cosmetic and pharmaceutical research purposes, Poieskin® is composed of a dermis – manufactured by layering type I collagen and primary human fibroblasts – on which primary human keratinocytes are printed to form a multi-layered epidermis. As a result, we were able to:
- Identify early critical bioprinting process parameters (CPP) and critical quality attributes (CQA) of bioprinted tissues as well as specific process analytical technologies (PAT) to ensure manufacturing stability and performance and to guarantee that what is produced/printed is what is designed (see Bioprinting Process Mapping in Figure 2
Tissue manufacturing by bioprinting. and Boxes 1 & 2);
- Assess the impact of bioprinting parameters and the environment on tissue functionality and manufacturing costs;
- To identify, develop and validate under industrial production conditions the various bioprinting solutions.
In addition, this allows us to highlight the main requirements that bioprinting still has to fulfil in order to tackle the obstacles surrounding TEP manufacturing and commercialization.
| Box 1. Bioprinting critical parameters. |
|---|
|
Improvement of standardization of TEP manufacturing processes
Concerning standardization of tissue manufacturing processes, in addition to the contamination prevention, the management and traceability of reagents and consumables used as raw materials (cells, biomaterials, culture media, flanges, inserts, etc.), these requirements concern more specifically:
- Full automation and robotization of bioprinting steps to minimize the divergence of certain CPPs (see Box 1) related to bioink formulation or substrate and printhead positions, and to ensure the stability of the CQAs related to cell deposition (see Box 2);
- The development of single cell bioprinting technologies to precisely control CQAs related to cell deposition such as the number and position of printed cells (per drop, per layer and within the multilayer structure);
- The integration of in-line imaging tools (with cellular resolution) acting as PAT (in addition to temperature, hydrometry and pH sensors) to monitor the fidelity of the printed structure to the CAD pattern via the measurement of the divergence of CQAs like as the number and position of the printed cells (per drop, per layer and within the multilayer structure), the droplet size, the presence of satellites;
- The integration of bioprinting technologies in closed systems, such as isolators, perfectly sterile and free of any particle likely to contaminate the bioprinted tissue and alter its growth during maturation and functionality, and/or infect the patient after implantation of the tissue. This integration should be accompanied by a demonstration of the absence of effect of the printing process on the tissue environment and namely particle generation, for example during printhead translation;
- The demonstration of regulatory compliance, which includes: i) the qualification of bioprinting machines (in agreement with GAMP & GMP/Pharmaceutical Equipment validation and in line with the drafting standard on Cell Therapy Manufacturing Equipment within ISO/TC 276: Biotechnology [17]) and software (GAMP/Compliant GxP Computerized Systems); ii) the validation of all stages of the process (regarding GMPs); as well as iii) the implementation of an adapted Quality Management System;
- The implementation of quality-by-design methodologies adapted to tissue development using bioprinting. This should make it possible to create design spaces specific to the process steps with the goal to adapt the process parameters to the inherent variability of the cells in order to obtain products of constant quality;
- The development of bioprinting systems compatible with standard cell culture devices and consumables (and not the reverse);
- The integration of bioprinting solutions into a complete biomanufacturing production line in which the bioprinting hardware and software solutions would be connected to other solutions to perform the upstream stages of extraction and cell culture, and packaging of the finished product downstream.
| Box 2. Critical Quality Attributes of bioprinted tissues. |
|---|
|
Improvement of TEP functionality
Improving tissue functionality relies on the capacity to reproduce the complexity of native human tissues (i.e., the spatio-temporal distribution of cells and biochemical, mechanical and physical stimuli that control the cellular micro-environment and govern cellular behaviour and tissue function). To address such a challenge involves more specifically to develop:
- Multimodal bioprinters integr-ating all the different bioprinting techniques (bio-extrusion, inkjet, laser, etc.) in order to take advantage of the performance of each of them (e.g., the deposition of viscous biomaterials by bioextrusion or micro-valve bioprinting and cell micro-patterning by laser-assisted bioprinting);
- Multicellular bioprinters to facilitate the production of complex tissues composed of several cell types;
- Single-cell bioprinting technologies to precisely control cell-cell interactions at the cellular level;
- CAD software for 4D bioprinting to design anisotropic, discontinuous tissue constituent patterns and program the tissue construct evolution that occurs by self-organization [18] (including shape evolution, cell proliferation and differentiation, ECM remodelling, etc.) during maturation and/or after implantation as a result of host–tissue interactions;
- Appropriate cell culture conditions and printing patterns to take into account the needs arising from the combination of different cell populations. This may also imply to consider the bioprinting sequence when the tissue is manufactured in several steps and/or several days (e.g., Poieskin® for which keratinocytes are printed 5 days after the dermis is printed).
Improvement of the cost–effectiveness & profitability of TEP treatments
With regards to improving the cost-effectiveness and profitability of TEP treatments, requirements for bioprinting deal with:
- Improving cell bioprinting yield (a certain number of cells remaining in cartridges) in order to minimize the cost of goods and cell preparation;
- Improving bioprinter throughput;
- Reducing costs of bioprinting devices without sacrificing critical process parameters;
- The integration of bioprinting technologies into closed systems, such as isolators, in order to reduce costs of infrastructure installation and ownership (especially for grade B clean rooms), what can be accompanied by a low availability of facilities due to their immobilization during cleaning and decontamination to avoid cross-contamination between two productions;
- The automation and robotization of processes in order to reduce the costs of specialised labor required to carry out preparation and manufacturing operations as well as quality control of individual batches and/or small volumes;
- Exploitation of the ability to pattern tissue components to shorten tissue maturation before implantation [19], and thus move towards the fabrication of off-the-shelf products;
- The development of modular systems designed to promote the continuous evolution of machines and compatible with the manufacture of multiple tissue types to allow economies of scope in production units;
- Optimizing the commonality of preclinical and clinical bioprinting platforms to facilitate the comparability of results obtained in different manufacturing sites and at different stages of TEP development and evaluation.
Next evolutions of bioprinting systems
We have recently taken into account these requirements with the development of a new bio-printing platform. This modular and multimodal platform integrates a 6-axis robotic arm as well as most of the different bioprinting techniques (bio-extrusion, microvalve and laser-assisted bioprinting). The manufacturing process has been automatized and is controlled by a Programmable Logic Controller (PLC) connected to sensors and actuators. As an example, environmental data and images of printed cells can be acquired in line and processed at each layer to monitor CQAs, thus ensuring, with a cellular resolution, that what is designed is what is printed. To facilitate the translation of preclinical R&D results into clinical phases and accelerate the development and access to innovative therapies for patients, this platform has been implemented into two bioprinter models based on the same technological core: NGB-R, a bio-printer already on the market for research in biology and tissue engineering, and NGB-C, a clinical version integrated into a closed isolator system and designed to meet the regulatory requirements surrounding the production of ATMPs, which is currently under validation.
Conclusions
Bioprinting technologies, developed at the convergence of automation, biology and digital technology, provide major advantages in tackling the main challenges for the manufacture of tissue engineered ATMPs. These concern the improvement of the standardisation of manufacturing processes, tissue functionality and cost–effectiveness and profitability of TEP treatments.
In this context, novel high resolution bioprinting approaches – bringing the capacity to design, manufacture and control in-line tissue architecture with cell resolution – offer major advantages such as the precise control of many CQAs like the position and number of cells per drop, per layer or even within multi-layer structures.
Such advantages should also progressively benefit from the implementation of industry 4.0 principles into high-resolution bioprinting systems and processes, thus giving rise to an era of Tissue Digital Biomanufacturing. Indeed, the increase of in-line sensors, especially cell imaging sensors, associated with bioprinters and their combination with computer supervisory systems should allow a continuous exploitation of all data collected during preclinical development phases to Quality Control operations carried out during clinical batch production. These tools will therefore give the opportunity to monitor CPPs and CQAs in real time, and will provide an interesting opportunity to quickly remove non-compliant tissues, to correct manufacturing in process, and even to simulate in real time the impact of a defect on tissue fate using, for example, artificial intelligence algorithms. Finally, let us bet that this technology should also offer new opportunities in the field of regenerative medicine in terms of decentralised production [20] as well as for the personalization of TEP, thus making it possible to propose patient specific therapies (e.g., by integrating autologous cells and/or using anatomical data of a patient during tissue design).
Financial & Competing Interests Disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. edqm.eu: https://ww.edqm.eu/en/events/european-day-organ-donation-and-transplantation
2. European Commission. Organs, blood, tissues & cells in the EU (2016).
3. Tissue Engineering Market Worth $11.53 Billion By 2022 | CAGR: 13%: https://www.grandviewresearch.com/press-release/global-tissue-engineering-market
4. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260(5110): 920–6. CrossRef
5. Hollister SJ, Murphy WL. Scaffold Translation: Barriers Between Concept and Clinic. Tissue Eng. Part B Rev. 2010; 17(6): 459–74. CrossRef
6. Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming Challenges Facing Advanced Therapies in the EU Market. Cell Stem Cell 2016; 19(3): 293–7. CrossRef
7. Guillemot F, Mironov V, Nakamura M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B’09). Biofabrication 2010; 2(1): 010201. CrossRef
8. Groll J, Boland T, Blunk T et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication 2016; 8(1): 013001. CrossRef
9. Moroni L, Boland T, Burdick JA et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018; 36(4): 384-402. CrossRef
10. Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. PNAS 2016; 113(12): 3179–84. CrossRef
11. Ong SC, Fukunishi T, Zhang H et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017; 7: 4566. CrossRef
12. Taniguchi D, Matsumoto K, Tsuchiya T et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Int. CardioVasc. Thor. Surg. 2018; 26(5): 745–52. CrossRef
13. organovo.com: https://organovo.com/tissues-services/exvive3d-human-tissue-models-services-research/ CrossRef
14. poietis.com: https://www.poietis.com/en/poieskin/welcome.php#poieskin1 CrossRef
15. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014; 32: 773–85. CrossRef
16. Ozbolat IT, Moncal KK, Gudapati H. Evaluation of bioprinter technologies. Add. Manufact. 2017; 13: 179–200. CrossRef
17. Lin-Gibson S, Hanraban B, Matosevic S, Schnitlzer A, Zhang J, Zylbergerg C. Points to consider for cell manufacturing equipment and components. Cell Gene Ther. Ins. 2017; 3(10): 793–805. CrossRef
18. Sasai Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 2013; 493: 318–26. CrossRef
19. McAllister TN, Maruszewski M, Garrido SA et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. The Lancet 2009; 373(9673): 1440–6. CrossRef
20. Harrison RP, Ruck S, Rafiq QA, Medcalf N. Decentralised manufacturing of cell and gene therapy products: Learning from other healthcare sectors. Biotechnol. Adv. 2018; 36(2): 345–57. CrossRef
Affiliations
Fabien Guillemot†, Laurence Hutter, Bruno Brisson, Delphine Fayol & Bertrand Viellerobe Poietis, Bioparc Bordeaux Métropole, 27 Allée Charles Darwin, 33600, France
†Corresponding author: fabien.guillemot@poietis.com