Residual DNA testing: Homebrew vs off-the-shelf solutions: INFOGRAPHIC
Cell & Gene Therapy Insights 2023; 9(7), 1009
10.18609/cgti.2023.128
Published: 17 August 2023
Infographic
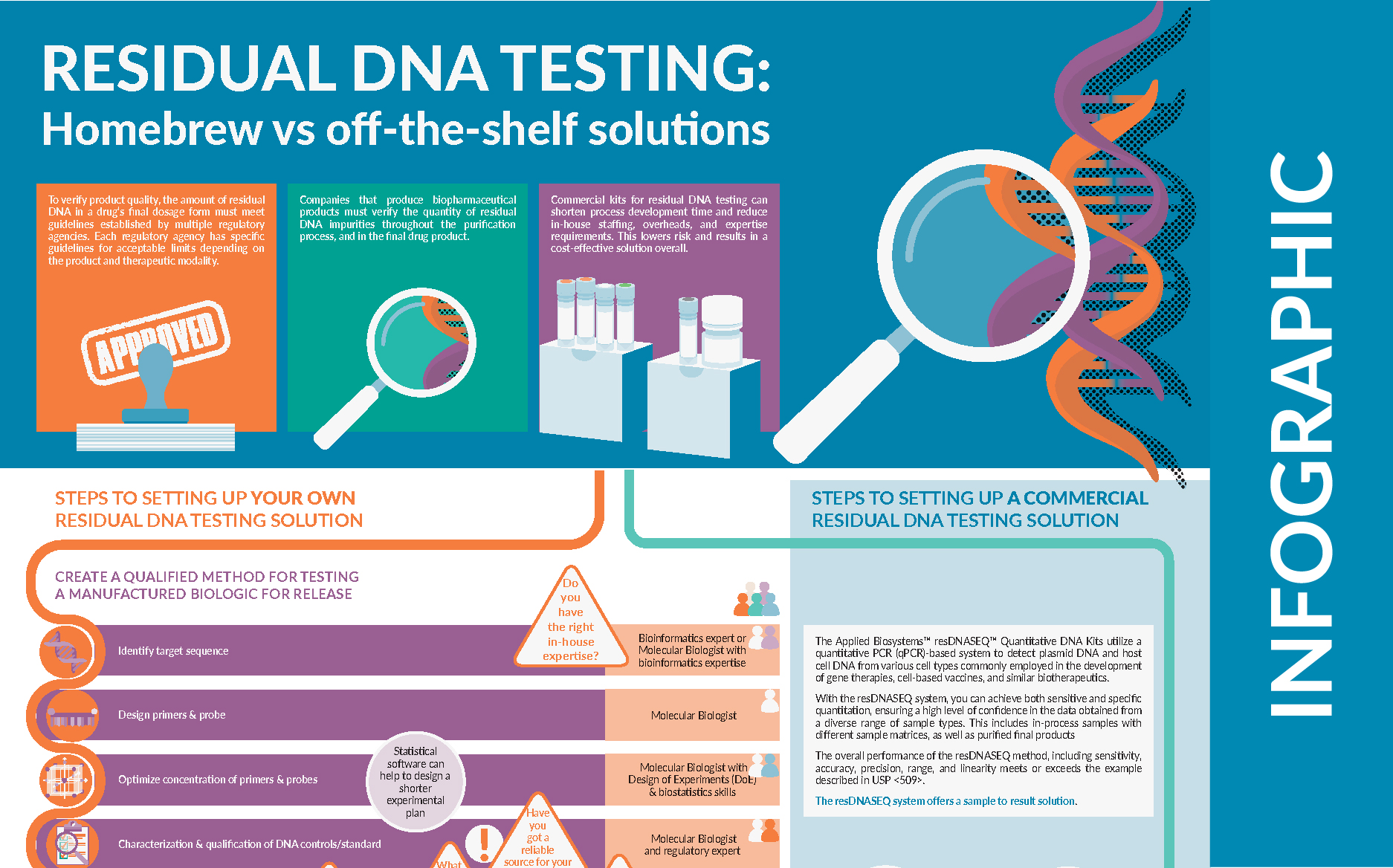 | To verify product quality, the amount of residual DNA in a drug’s final dosage form must meet guidelines established by multiple regulatory agencies. Each regulatory agency has specific guidelines for acceptable limits depending on the product and therapeutic modality. This infographic demonstrates the steps, considerations and significant challenges involved in developing your own residual DNA testing solution. Commercial kits for residual DNA testing can shorten process development time and reduce in-house staffing, overheads, and expertise requirements. This lowers risk and results in a cost-effective solution overall. |
