Development of purification steps for several AAV serotypes using POROS™ CaptureSelect™ AAVX affinity chromatography
Cell Gene Therapy Insights 2018; 4(7), 637-645.
10.18609/cgti.2018.061
Genethon is an integrated R&D centre created in 1990 by the French Association of Muscular Dystrophy. The Centre performs both gene therapy translational research and clinical development, with a specific focus on rare genetic diseases such as neuromuscular disorders, immuno-deficiencies, eye diseases and liver diseases. The two main vector platforms used at Genethon are AAV and lentiviral vectors.
Current purification methods for viral vector manufacturing usually involve multiple steps of chromatography, for example ion exchange and hydrophobic interaction, which leads to cumulative yield loss and ultimately, increased cost.
Thermo Fisher’s CaptureSelect™ technology addresses this issue by providing high-affinity binding ligands for affinity chromatography. This approach provides high purity and productivity in fewer steps than traditional purification processes, while also offering process consistency and scalability.
The technology is based on the utilisation of the variable domains of camelid heavy-chain-only antibodies (VHH) which offer full functionality in antigen-specific recognition and also high-affinity binding (the three CDR regions of the VHH ligand provide unique, tunable specificity). Due to their compact structure, the domains are highly robust and capable of withstanding the various conditions typical of chromatography runs. Additionally, the selected ligands have the advantage of being produced in an animal origin-free system – Saccharomyces cerevisiae (baker’s yeast).
The result is a unique screening technology for target specificity, mild elution and stability, which is designed for commercial purification processes.
The CaptureSelect ligand is immobilized on rigid poly[styrene divinylbenzene] Thermo Scientific™ POROS™ particles, producing affinity resins that are suitable for process-scale bioseparations. POROS™ chromatography resins are characterized by large through pores with increased surface area, resulting in higher capacity that can be maintained at high flow rates, making them suitable for purification of large biomolecules like viral vectors.
POROS™ and CaptureSelect™ technologies were combined in order to develop and commercialise 3 affinity resins, specifically engineered for AAV vector purification: initially, POROS™ CaptureSelect™ AAV8 and AAV9 resins were launched, followed by POROS™ CaptureSelect™ AAVX, which enables purification of a broad range of serotypes, both naturally-occurring and synthetic.
In all three cases, the ligands are immobilized on the high performing POROS™ R50 base bead. POROS™ CaptureSelect™ AAV resins offer superior specificity and capacity for the purification of many different AAV serotypes, resulting in high purity and yield in one step from crude material (frequently then followed by a concentration step). This significant simplification of the purification process – combined with a reduction in unit operations due to the high specificity of the resin – enables the large-scale development of gene therapy vectors and reduces cost of goods (CoG).
POROS™ CaptureSelect™ AAV products also come with full Regulatory Support Files (RSF) paving the way for implementation in commercial manufacturing
Screening affinity resins at small scale
In this example, screening was performed using the four affinity resins that are available on the market for the purification of AAV vectors: AVB Sepharose, POROS™ CaptureSelect™ AAV8, POROS™ CaptureSelect™ AAV9, and POROS™ CaptureSelect™ AAVX. Different AAV serotypes from clarified harvest were used for the screening: from conventional serotypes AAV8, 9 and 10, to eight different synthetic serotypes resulting from capsid shuffling and/ or chimerisation.
These experiments were performed in 96 well-plates format using a small amount of each resin (25µl). To ensure the quality of results, up to three different load quantities of AAV were tested, ranging from 1e11 to 2.5e12 VG/ml of resin. The binding efficiencies of the different resins were calculated by estimating the titer in the flow-through compared to the titer loaded onto the resin.
Figure 1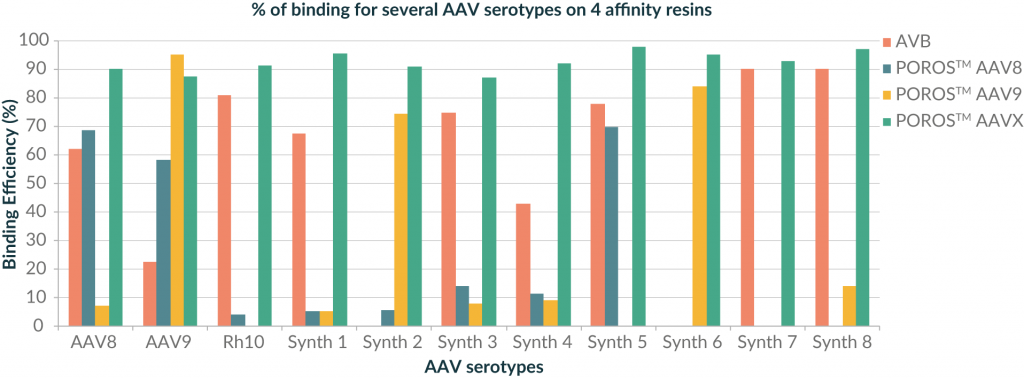
The POROS™ CaptureSelect™ AAV8 and AAV9 resins clearly demonstrated affinity for AAV8 and AAV9 serotypes, but POROS™ CaptureSelect™ AAVX resin also demonstrated a strong affinity for both serotypes. For the other AAV serotypes, the pattern of binding to the different resins is generally variable. However, for each of the different serotypes tested, the binding efficiency to the POROS™ CaptureSelect™ AAVX resin is higher than 80%, demonstrating a high binding capacity and extremely broad selectivity to both naturally occurring and synthetic capsids.
Operating conditions & the performance of POROS™ CaptureSelect™AAVX resin for the purification of conventional serotypes AAV8 & AAV9
The next step in evaluating whether POROS™ CaptureSelect™ AAVX could be used as a platform resin for purification of multiple serotypes involved evaluating capacity compared to residence time: 400 column volume of clarified harvest was loaded with three different residence times applied, varying from 0.8 to 2.4 min.
Beginning with AAV8, Figure 2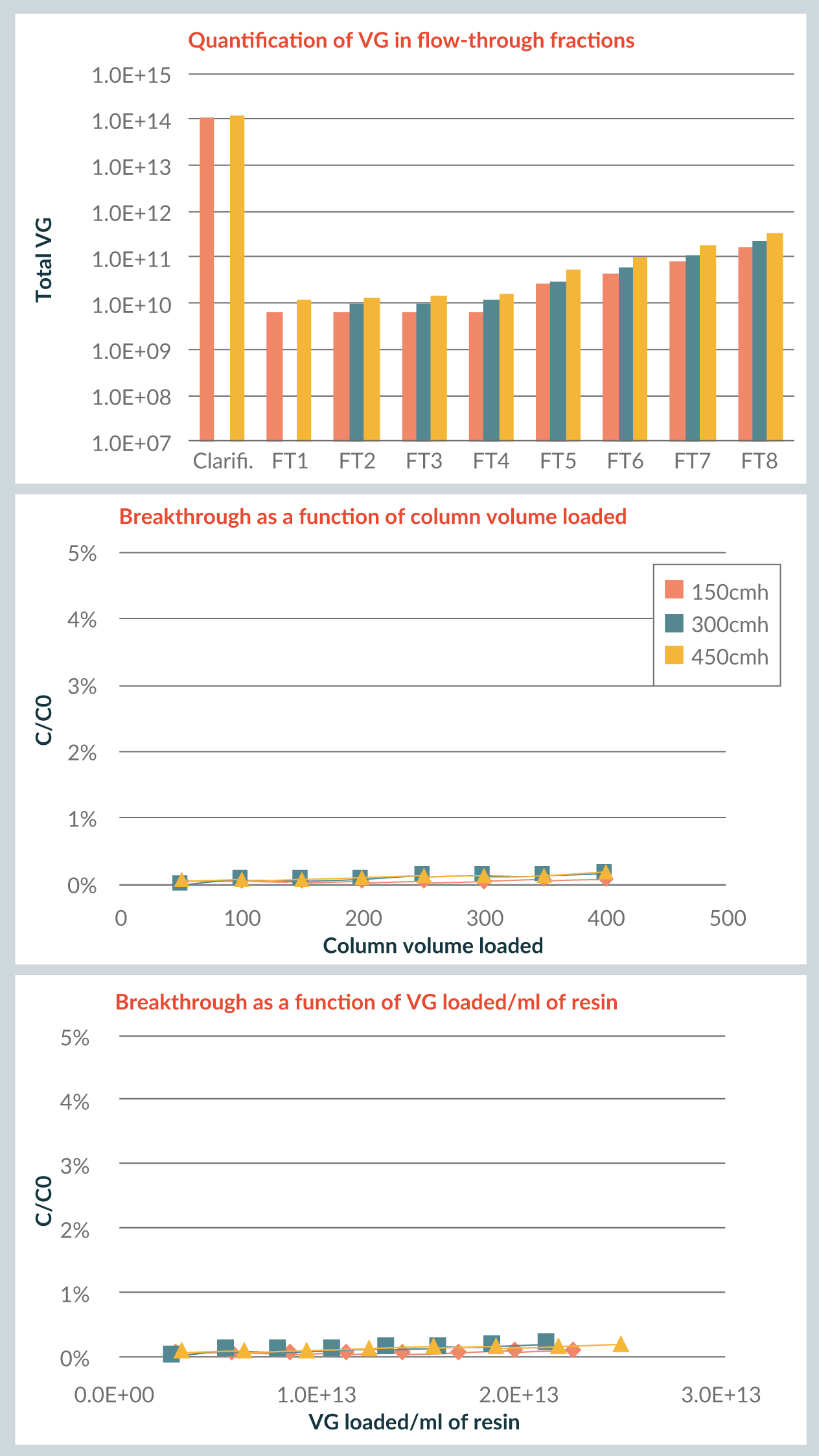
One associated benefit is that it provides a high degree of process design flexibility: one can design a step where the loading phase lasts just a few hours, or one can also perform a long load – for example, running a POROS™ CaptureSelect™ AAVX step overnight. In addition, the resin’s very high affinity allows for accommodation of feed variations without loss of product in flow-through.
Elution recovery was above 80% at each of the three different flow rates used Figure 3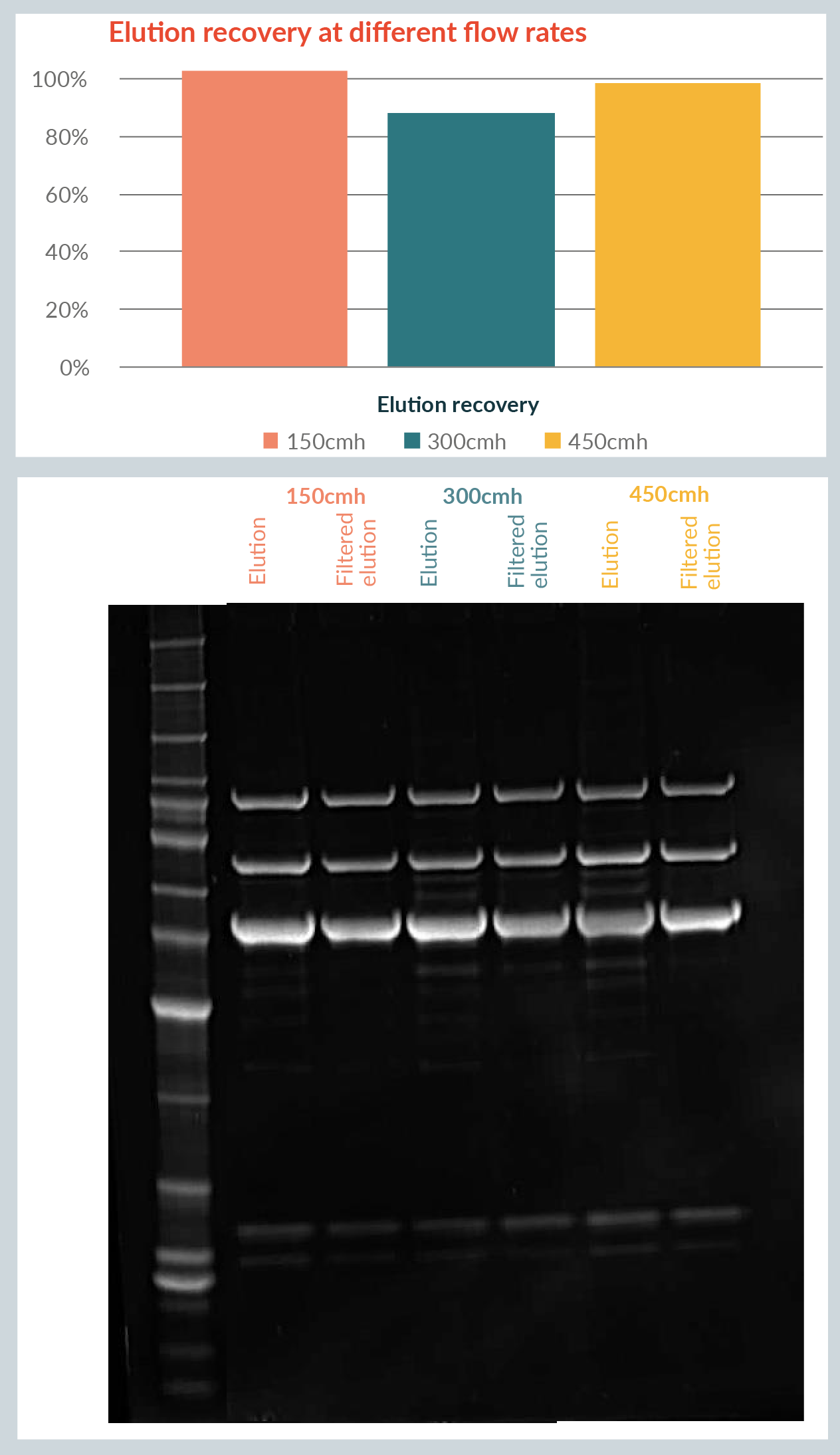
To increase the purity of the AAV8 before filtration and avoid precipitate in the purification process, a washing strategy was developed for the purification of AAV8 on the POROS™ CaptureSelect™ AAVX resin. Several wash conditions combining varying pH levels and different additives were screened and evaluated for reduction in contaminant levels and elution precipitation.
This washing strategy was then compared to a control run on POROS™ CaptureSelect™ AAVX resin without wash. In addition, this condition on POROS™ CaptureSelect™ AAVX resin was compared to the current condition on POROS™ CaptureSelect™ AAV8 resin (which has a wash design of its own). This experiment used 2.5 minutes residence time and ~1,8e13 VG/ml of resin.
Figure 4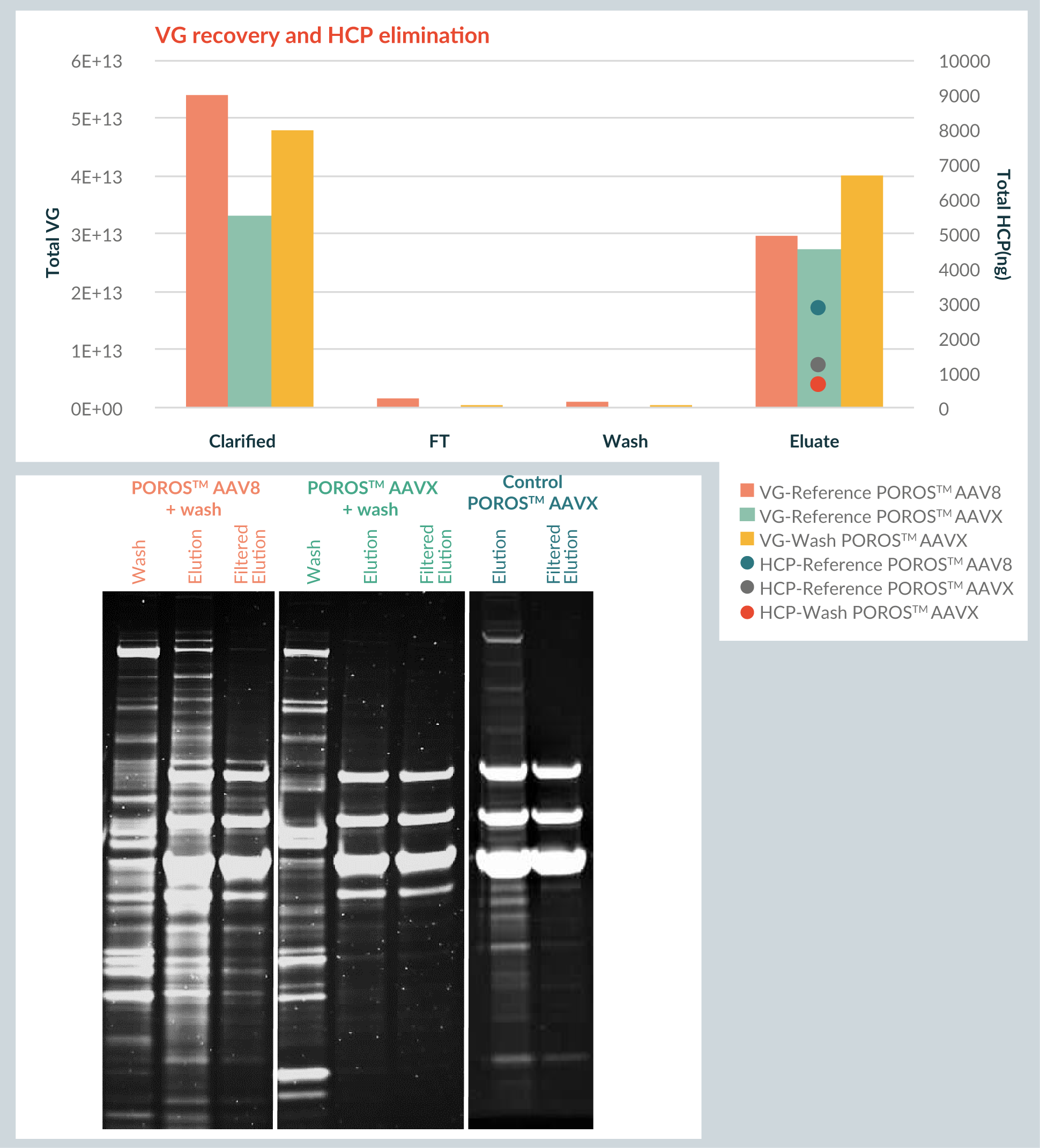
Moving on to AAV9 viral vector, the first step again involved evaluating capacity versus flow rate. Similar results were obtained, demonstrating that residence time had no effect on performance as it relates to capacity.
However, when the same wash conditions used for the AAV8 experiment were applied to AAV9, AAV9 was released from the column, illustrating that the binding of AAV9 viral vector to the POROS™ CaptureSelect™ AAVX resin is less strong than that of AAV8. (This is in correlation with previous observations that the binding of AAV9 viral vector to the POROS™ CaptureSelect™ AAV9 resin is also less strong than that of AAV8 viral vector to the POROS™ CaptureSelect™ AAV8 resin). Therefore it was necessary to design a new specific wash for AAV9 viral vector. The evaluation of different wash pH levels and additives was consequently repeated.
The resulting new condition was evaluated with POROS™ CaptureSelect™ AAVX resin (again, tested both with and without wash step) for AAV9 vector. It was also compared to the utilisation of POROS™ CaptureSelect™ AAV9 resin without wash, which is the standard for that particular resin. The load quantities and residence time were similar to the AAV8 experiment (2.5 minutes residence time and ~1,8e13 VG/ml of resin).
Figure 5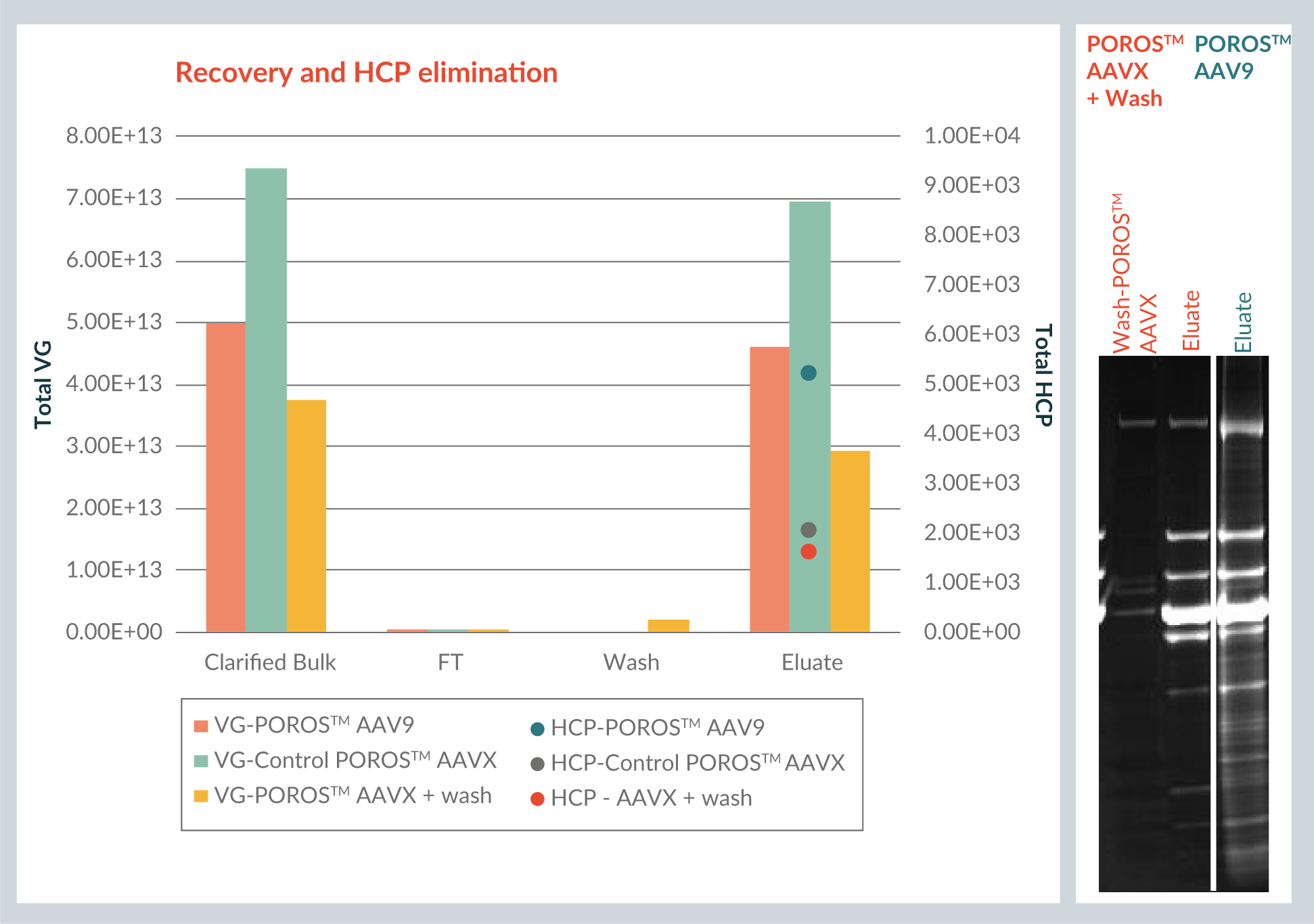
For the POROS™ CaptureSelect™ AAVX resin itself, the wash has little effect on the HCP level. However, when comparing the pattern of the eluate between the POROS™ CaptureSelect™ AAVX resin including wash and the POROS™ CaptureSelect™ AAV9 resin eluate, it is evident that POROS™ CaptureSelect™ AAVX resin provides a cleaner profile by comparison.
Utilisation of POROS™ CaptureSelect™ AAVX resin for the purification of a synthetic AAV serotype
POROS™ CaptureSelect™ AAVX affinity resin was evaluated next for the purification of a synthetic serotype obtained by chimerisation. The same load and flow rate conditions that were proven to work very well for AAV8 and AAV9 viral vectors were applied. However, evaluation of capacity versus flow rate was not repeated, and in the first step, a wash was not applied because wash conditions should be defined more specifically.
Figure 6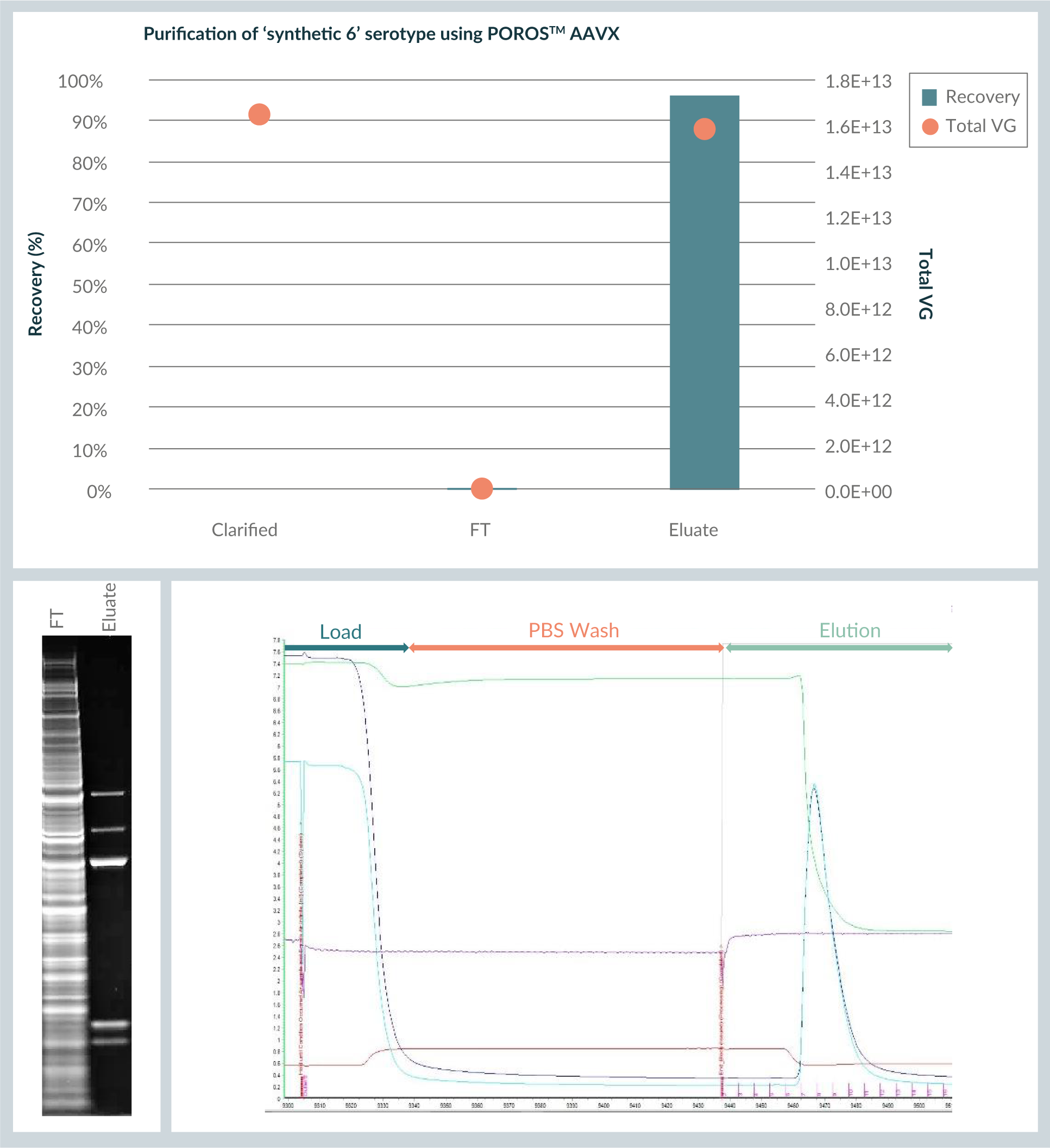
To conclude, this data shows that POROS™ CaptureSelect™ AAVX affinity chromatography resin binds a broad range of serotypes and this was demonstrable at small scale (through 96 well-plate experiments).
With regard to operating conditions, POROS™ CaptureSelect™ AAVX resin is shown to be very flexible, demonstrating utility with a broad range of flow rates and load quantities, which in turn allows for flexibility in process design and also accommodation of potential feed variations.
These data indicate that the POROS™ CaptureSelect™ AAVX resin is usable as a platform resin for AAV purification as, for example, load elution and flow rate conditions are broadly applicable directly, without the need for optimisation for different serotypes (including for synthetic ones).
Another observation is that the affinity or interaction strength varies from one serotype to another – for example, when we compare AAV9 and AAV8 vectors. Because of this, if one wishes to apply wash conditions in particular to avoid the formation of precipitate and to increase purity, then those conditions need to be determined specifically for each serotype.
Finally, these experiments also demonstrate that the purity of the products and HCP removal are improved on the POROS™ CaptureSelect™ AAVX affinity resin, as compared to the former generation POROS™ CaptureSelect™ AAV affinity resins.
With all of these properties in mind, POROS™ CaptureSelect™ AAVX affinity chromatography resin has been demonstrated to be a good candidate for use as a platform resin – i.e. as a single reference for the purification of a broad range of different AAV serotypes.
Financial & competing interests disclosure
The author has no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties.
This article has been written based on a webinar presentation by Dr Magali Toueille for Cell and Gene Therapy Insights, the on demand version of which can be accessed here free of charge.
Affiliations
Dr Magali Toueille, Head of Preclinical Production & Downstream Process Development, Genethon
Ludivine Dejoint, Genethon
Esther Attebi, Genethon
Jessica Cartigny, Genethon
Céline Rasle, Genethon
Stéphanie Potier, Genethon
Stéphanie Rundwasser, Genethon
Laurence Guianvarc’h, Genethon
Christine Lebec, Genethon
Matthias Hebben, Genethon
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</