Unleashing potential: tackling CAR-T cell production challenges to support the treatment of blood cancer
Cell & Gene Therapy Insights 2024; 10(1), 149–157
DOI: 10.18609/cgti.2024.022
CAR-T cell therapy is a ground-breaking and highly effective personalized treatment involving T cells from a patient’s immune system, which are genetically engineered to target and destroy cancer cells. CAR-T cell therapy was considered as a last-line treatment option for patients who meet specific criteria. However, clinical trials demonstrated that these therapies can be used and recommended as a second line treatment for whose cancer has proven resistant to traditional therapies like chemotherapy or radiation or when a patient’s illness recurs. As of 2023, CAR-T cell therapy is approved for use in individuals with some types of non-Hodgkin lymphomas, B-cell acute lymphoblastic leukemia, and multiple myeloma. While CAR-T cell therapy has shown remarkable clinical success in treating specific blood cancers, current manufacturing processes have significant room for improvement.
Introduction
CAR-T cell therapy is a ground-breaking and highly effective personalized treatment involving T cells from a patient’s immune system, which are genetically engineered to target and destroy cancer cells [1].
CAR-T cell therapy was considered as a last-line treatment option for patients who meet specific criteria. However, clinical trials demonstrated that these therapies can be used and recommended as a second line treatment for whose cancer has proven resistant to traditional therapies like chemotherapy or radiation or when a patient’s illness recurs [2] [3] [4].
As of 2023, CAR-T cell therapy is approved for use in individuals with some types of non-Hodgkin lymphomas, B-cell acute lymphoblastic leukemia and multiple myeloma. Eligibility for CAR-T cell therapy is determined based on the nature of the disease and the patient’s health status.
A challenging and unique production process
While CAR-T cell therapy has shown remarkable clinical success in treating specific blood cancers, current manufacturing processes have significant room for improvement. The CAR-T cell therapy process follows a multi-stage system that typically spans several weeks. The individual stages of production for these therapies, that are all products in the market autologous therapies, can also be challenging and complex, requiring strict quality checks and short timelines [2]Bloodcanceruk.org.uk (accessed Nov 2023). [5]Ramos CA, Heslop HE, Brenner MK. CAR-T cell therapy for lymphoma. Annu. Rev. Med. 2016; 67, 165–183. [6]Geethakumari PR, Ramasamy DP, Dholaria B, et al. Balancing quality, cost, and access during delivery of newer cellular and immunotherapy treatments. Curr. Hematol. Malig. Rep. 2021; 16(4), 345–356. [7]. The main steps involve:
Leukapheresis
After drawing blood from the patient, white blood cells are separated from the red blood cells and other components. The white blood cells contain the T cells that will be crucial for later CAR-T cells development. Once white blood cells are collected, the remaining blood is reinfused back to the patient.
T cell activation, transduction, and expansion
From the patient collected white blood cells, T cells are purified and undergo meticulous genetic modification, which progresses through activation, transduction, and expansion phases. Viral vectors (lentivirus and/or retrovirus) are key in introducing the desired genetic material into T cells, converting them into potent CAR-T warriors.
Formulation and transport
Activated and expanded CAR-T cells are then formulated in adapted media for injection, transforming into a therapeutic elixir poised to combat blood cancers. The final product is only released after being checking against stringent quality control standards.
Regarding transportation, in the case of centralized manufacturing, there may be logistical concerns with cryopreservation or shipping of the final product. In contrast, decentralized manufacturing models allow to minimize the risk of shipping and stability of the product due to cryopreservation.
CAR-T cells infusion
The enhanced CAR-T cells return to the clinical team and are administered to the patient via intravenous drip. This transformative infusion marks the culmination of a complex, weeks-long process. Now, the therapy is ready to unleash its potential within the patient’s body.
One of the most crucial factors affecting patient outcomes in CAR-T cell therapy is the ‘vein-to-vein’ time, which refers to the duration between the collection of T cells (leukapheresis) and the infusion of the CAR-T product.
For all US FDA-approved products, it takes three to five weeks for manufacturing and quality assessment to be conducted before the product is ready for use. CAR-T cell products available in the market are manufactured in a specific centralized manufacturing facility, aiming for a turnaround time of 16–33 days. However, this timeframe is susceptible to delays, and failure rates range from 1 to 18% [8]Elsallab M, Maus MV. Expanding access to CAR T cell therapies through local manufacturing. Nat. Biotechnol. 2023; 41(12), 1698–708. .
Prolonged manufacturing times have been correlated with a potential decline in CAR-T cell potency, as indicated in studies such as the one conducted by Saba Ghassemi et al. in 2018 [9]Ghassemi S, Nunez-Cruz S, O’Connor RS, et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 2018; 6(9), 1100–9. . This decline in potency raises concerns about compromising the therapeutic impact of the administered CAR-T cells.
Alternatively, the ‘vein-to-vein’ time is also influenced by other steps such as shipping to the site of patients, regulatory testing, insurance processes, and broader operational facets, of all whom collectively hold substantial implications for patients with progressive diseases.
The waiting period often imposes patients to undergo additional bridging therapies, which can increase the risk of side effects and complications. Delivering the treatment to the patient as quickly as possible is, therefore, critical because the patient’s clinical status could deteriorate very rapidly, and any delay could harm the patient’s chances of survival. The current duration for ‘vein to vein’ time is problematic, particularly for patients in the advanced stages of the disease, as it can impact their eligibility for CAR-T therapy (Figure 1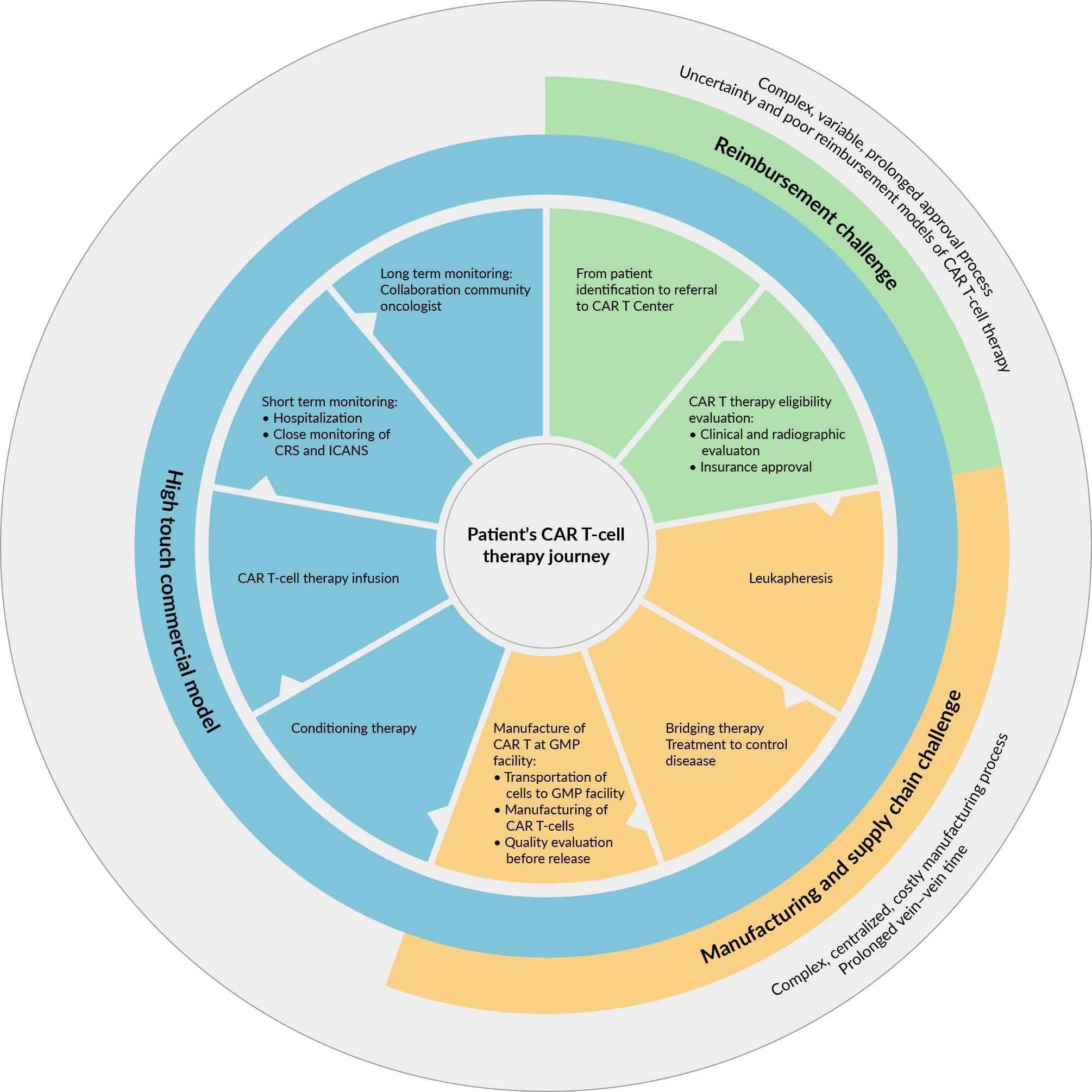 CAR-T cell therapy cycle and associated challenges.) [10] [11]Lutfi F, Moaath Mustafa A, Siglin J, et al. The impact of bridging therapy prior to CAR-T cell therapies clincial outcomes of patients with relapsed refractory large B-cell lymphoma. Tct.fonfex.com. Feb 8, 2021; (accessed Nov 2023)..
CAR-T cell therapy cycle and associated challenges.) [10] [11]Lutfi F, Moaath Mustafa A, Siglin J, et al. The impact of bridging therapy prior to CAR-T cell therapies clincial outcomes of patients with relapsed refractory large B-cell lymphoma. Tct.fonfex.com. Feb 8, 2021; (accessed Nov 2023)..
Because of all the CAR-T products are autologous, another critical parameter is the chain of identity. To maintain the chain of identity, labelling and tracking of material, from leukapheresis collection all the way through CAR-T cells administration, must be extremely well documented to avoid the administration to patient of a wrong batch of CAR-T product.
The importance of safety testing in CAR-T
In the production of CAR-T cell therapies, multiple tests are conducted throughout the process, from the early stages to the final release of the treatment. It is crucial to prioritize safety to minimize the risks associated with these therapies, particularly contamination. These essential tests include mycoplasma testing, sterility testing, and bioburden testing, environmental monitoring, and endotoxin detection [12]Gebo JET, Lau AF. Sterility testing for cellular therapies: what is the role of the clinical microbiology laboratory? J. Clin. Microbiol. 2020; 58(7), e01492–19. [13]Totten AH, Adams AJ, Halas HK, et al. Comparison of five commercial molecular assays for mycoplasma testing of cellular therapy products. J. Clin. Microbiol. 2023; 61(2), e0149822. [14] [15].
Mycoplasma testing [12]
Mycoplasma testing is a critical evaluation to detect the presence of mycoplasma, a type of bacteria that can contaminate cell cultures and potentially affect the quality and safety of therapeutic products. Ensuring the absence of mycoplasma is essential to maintain product integrity and patient safety;
Mycoplasma contamination poses a significant challenge in biologics development and production, potentially compromising cellular products and the safety of biopharmaceuticals. Regulatory agencies worldwide mandate mycoplasma testing during development and manufacturing. Biopharmaceutical companies conducting mycoplasma testing must adhere to Chapter USP <63> Mycoplasma Tests by the United States Pharmacopeia (USP), and European Pharmacopoeia (EP) Chapter 2.6.7 [16] [17];
The traditional method has a turnaround time of 28 days (Figure 2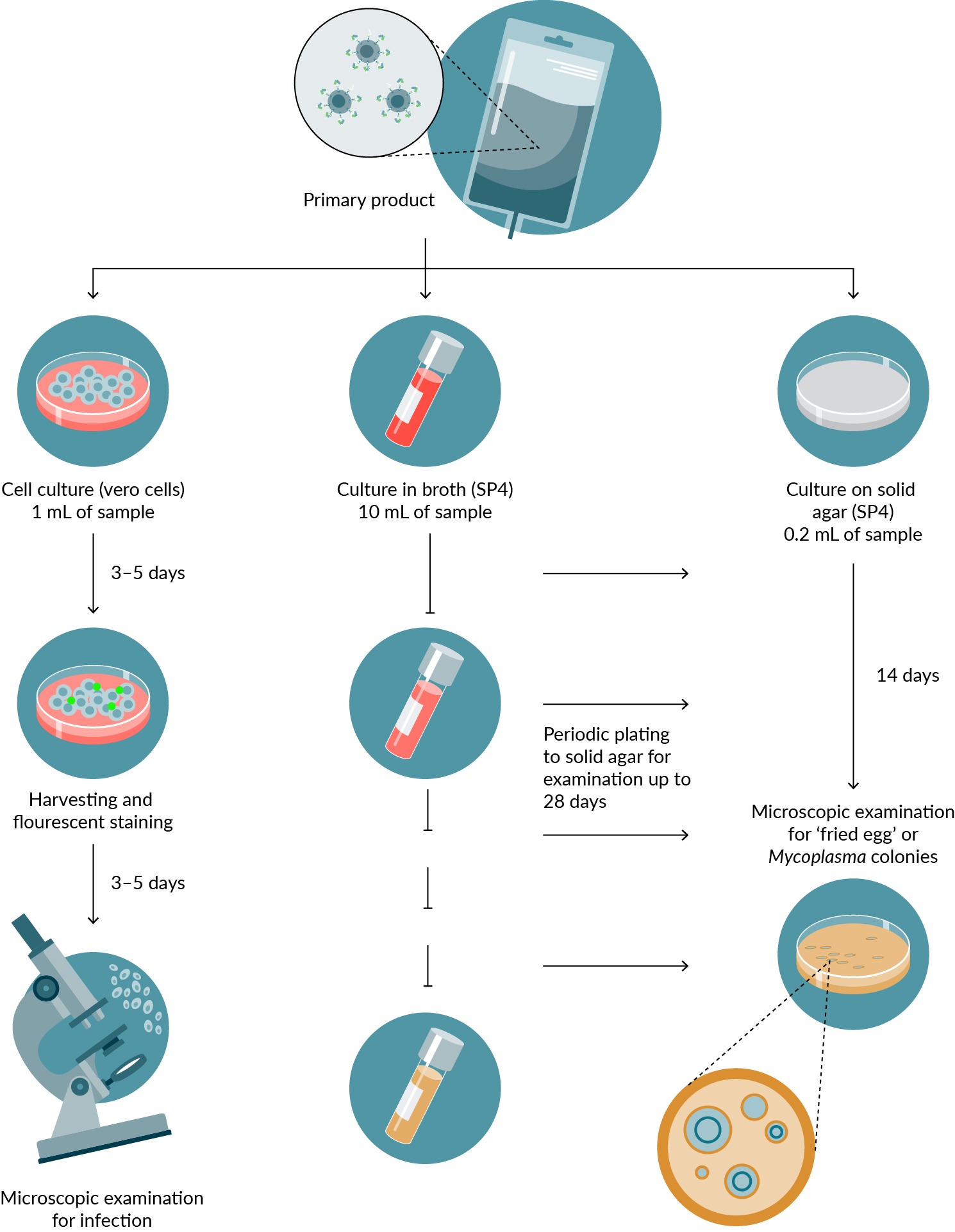 Traditional methods with turnaround time of 28 days.The mycoplasma analysis for cell and gene therapy products is a traditional testing method that follows specific requirements. The primary product undergoes analysis through three methods: 1) Culture on cells with mycoplasma contamination assessed via fluorescence microscopy. 2) Culture of the primary product in medium followed by subculture on permissive solid agar. 3) Direct culture onto solid agar, incubated for 14 days and examined for distinctive mycoplasma colonies.). Making this test critical. A nucleic-acid test-based methods are allowed and give results in one hour to few hours.
Traditional methods with turnaround time of 28 days.The mycoplasma analysis for cell and gene therapy products is a traditional testing method that follows specific requirements. The primary product undergoes analysis through three methods: 1) Culture on cells with mycoplasma contamination assessed via fluorescence microscopy. 2) Culture of the primary product in medium followed by subculture on permissive solid agar. 3) Direct culture onto solid agar, incubated for 14 days and examined for distinctive mycoplasma colonies.). Making this test critical. A nucleic-acid test-based methods are allowed and give results in one hour to few hours.
Sterility testing [11]:
Sterility testing involves assessing the absence of viable microorganisms in the product and verifying that it is free from any potentially harmful bacteria, yeasts, or molds;
Sterility is paramount in the manufacturing of CAR-T cell therapies. Any microbial contamination can lead to severe adverse patient reactions, making sterility testing critical for product safety;
Biopharmaceutical companies conducting sterility testing must adhere to Chapter USP <71> Sterility Tests by the United States Pharmacopeia (USP), and European Pharmacopoeia (EP) Chapter 2.6.1, which specify a turnaround time of no less than 14 days [18] [19]..However,alternative rapidmicrobiological methods are allowed to be used to reduce the sterility testing time.
These tests play a vital role in the quality control of CAR-T cell therapy manufacturing, ensuring that the final product is free from harmful contaminants, thereby maintaining the safety and effectiveness of the treatment.
A dynamic evolution in industrial processes
CAR-T cell therapies are fresh infusion products with a limited shelf life, presenting unique challenges for traditional microbiological testing methods (14-day sterility testing, 28-day mycoplasma testing), which were initially designed for less time-sensitive applications. To meet the urgent demand for expedited therapy delivery to patients, CAR-T cell therapy manufacturers have implemented an evolving industrial process that involves [20]Hort S, Herbst L, Bäckel N, et al. Toward rapid, widely available autologous CAR-T cell therapy—artificial intelligence and automation enabling the smart manufacturing hospital. Front. Med. (Lausanne) 2022; 9, 913287. :
Increasing digitalization and automation: this shift toward greater digitalization and automation is crucial to optimize production capacity, reduce costs, and generate valuable data;
Reducing turnaround time: manufacturers are looking to employ rapid methods and modularity to speed up the production process and time for release testing, ensuring that patients receive therapies more quickly;
Enhancing instrumentation: the simplification and improvement of instrumentation aims to reduce complexity in producing CAR-T cell therapies.
Taking a closer look at where CAR-T manufacturing processes could be further adapted, industry pioneers as Kite Pharma recently acknowledged the necessity for rapid quality control methods and emphasized the importance of early QC involvement in the design and development phase. Experts at the company have proposed early monitoring and development of methods with low invalid rates, alongside early investments in rapid methods and innovative lab-in-a-pouch technologies, for use in processes such as mycoplasma testing.
Experts from the Dana-Farber Cancer Institute also highlighted the necessity of mycoplasma testing in product release and the need to address current challenges encountered in ensuring product safety. In response, the institute is exploring testing methods that can be completed in less than 5 min, without advanced laboratory training, where results are available in under one hour and with a reduced risk of contamination.
The changing landscape of CAR-T cell therapy production comes in response to the increasing demand for swift and effective patient care. Driven by the urgency to provide timely access to these transformative treatments, researchers and industry leaders are refining manufacturing processes (e.g., ‘Cell Shuttle’ of Cellares, platform of OriBiotech) to optimize efficiency and ensure more patients can benefit from CAR-T cell Therapies promptly. With this commitment manufacturers are seeking to transform healthcare delivery, emphasizing scientific rigor and accessibility.
Addressing the skill gap in cell and gene therapy
A further challenge faced by manufacturers of these complex therapeutics is the current shortage of skilled workers in key areas that are critical to producing, testing, and delivering these innovative treatments. The shortage extends to manufacturing, analytical development, testing, and quality control roles [21].
One of the most significant barriers to addressing this workforce shortage is the cost of training. While hands-on laboratory training is essential for success in the field, it can also be prohibitively expensive. Both candidates aspiring to enter the industry and educators seeking to provide relevant training face challenges associated with the costs involved. Implementing automated equipment and instruments is one potential solution to bridge this workforce gap. The wider usage of these types of technological advancements could significantly aid the industry in addressing the shortage of skilled personnel and the associated training costs [20]Hort S, Herbst L, Bäckel N, et al. Toward rapid, widely available autologous CAR-T cell therapy—artificial intelligence and automation enabling the smart manufacturing hospital. Front. Med. (Lausanne) 2022; 9, 913287. .
What does the future potentially hold for CAR-T cell therapy?
The future of CAR-T cell therapy is marked by ongoing efforts to streamline and expedite safe, effective, quality treatment delivery to patients (Figure 3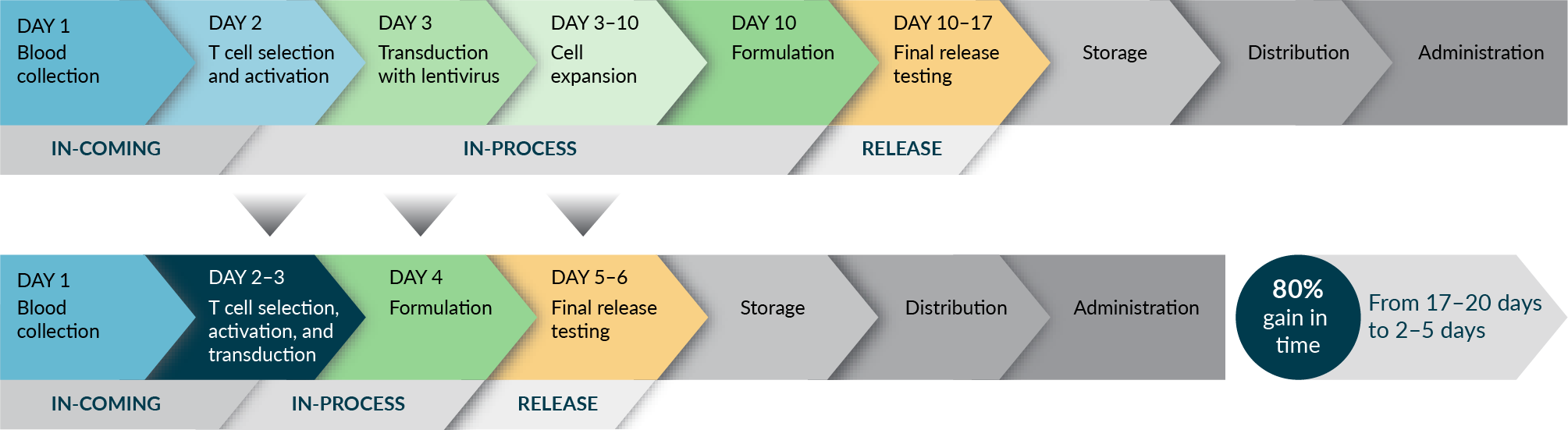 Trends: moving to next gen manufacturing.). Examples of successful implementations of shorter timelines could be given by University of Pennsylvania’s 3-day manufacturing process, Novartis’s T-charge, and next-day manufacturing platforms of Gracell Biotechnologies [22]Yang J, He J, Zhang X, et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study. Blood Cancer J. 2022; 12(7), 104. .
Trends: moving to next gen manufacturing.). Examples of successful implementations of shorter timelines could be given by University of Pennsylvania’s 3-day manufacturing process, Novartis’s T-charge, and next-day manufacturing platforms of Gracell Biotechnologies [22]Yang J, He J, Zhang X, et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study. Blood Cancer J. 2022; 12(7), 104. .
Manufacturers are actively exploring strategies to minimize the ‘vein-to-vein time’, but maintaining the efficacy of these product, and employ new solutions to combat other challenges, such as low production volume, short product shelf life, handling of complex raw materials, tracking and data integrity, scalability and patient demands [14]Biomerieux.com. With a complete portfolio of Quality Control solutions, bioMérieux ensures the safety of patients undergoing cell and gene therapies (accessed Nov 2023)..
Additionally, the expedited nature of shorter manufacturing requires heightened vigilance for:
Rapid sterility testing. Pharmacopeia are developing new chapters (USP <1071> [Rapid Microbial Tests for Release of Sterile Short-Life Products: A Risk-Based Approach], USP <72> [Respiration-Based Microbiological Methods for the Detection of Contamination in Short-Life Products], and EP 2.6.27 [Microbiological examination of cell-based preparations]) to help release short-shelf life products such as CAR-T cell therapies using modern rapid microbiological methods;
Potential lentiviral vector persistence, such as replication competent lentivirus testing;
And finally, control of vector copy number that can potentially alter expression of cellular genes and contribute to tumorigenicity.
A promising solution with the potential to provide cost-effective solutions [23]Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022; 21(1), 78. Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022; 21(1), 78. , lies, next-generation CAR-T Therapies in association with CRISPR-Cas9 genetic editing technology and in vivo induced CAR-T cells using nanoparticles loaded with mRNA’s coding for CAR genes [23]Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022; 21(1), 78. Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022; 21(1), 78. . Though in their early stages, preclinical data suggest that nano-delivery systems for in vivo CAR-T cells hold promise for optimizing their efficacy and overall cost [24]Xin T, Cheng L, Zhou C, et al.In vivo induced CAR-T cell for the potential breakthrough to overcome the barriers of current CAR-T cell therapy. Front. Oncol. 2022; 12, 809754. . Over the past decade, CAR-T cell therapy has made significant progress, with multiple products available for clinical use.
The industry’s focus on digitalization, automation and rapid testing methods reflects its commitment to providing patients with timely access to this life-saving treatment. As the field continues to evolve, CAR-T therapy holds promise as a transformative approach to cancer treatment, offering renewed hope to many patients who have exhausted other options.
Discover the bioMérieux’s proven portfolio of value-added solutions: www.biomerieux.com.
References
1. Biomerieux.com. What is CAR-T cell therapy? (accessed Nov 2023). Crossref
2. Bloodcanceruk.org.uk (accessed Nov 2023). Crossref
3. Cancerresearchuk.org (accessed Nov 2023). Crossref
4. Biomerieux.com. CAR-T cell therapy: for which indications? (accessed Nov 2023). Crossref
5. Ramos CA, Heslop HE, Brenner MK. CAR-T cell therapy for lymphoma. Annu. Rev. Med. 2016; 67, 165–183. Crossref
6. Geethakumari PR, Ramasamy DP, Dholaria B, et al. Balancing quality, cost, and access during delivery of newer cellular and immunotherapy treatments. Curr. Hematol. Malig. Rep. 2021; 16(4), 345–356. Crossref
7. Biomerieux.com CAR-T cell therapy—why is it challenging? (accessed Nov 2023). Crossref
8. Elsallab M, Maus MV. Expanding access to CAR T cell therapies through local manufacturing. Nat. Biotechnol. 2023; 41(12), 1698–708. Crossref
9. Ghassemi S, Nunez-Cruz S, O’Connor RS, et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 2018; 6(9), 1100–9. Crossref
10. Hua J, Baoxia D, Gao L, et al. Clinical results of multicenter study of the first-in-human dual BCMA and CD19 targeted novel platform fast CAR-T cell therapy for patients with relapsed/refractory multiple myeloma. ash.confex.com. Published December 5, 2020; (accessed Nov 2023). Crossref
11. Lutfi F, Moaath Mustafa A, Siglin J, et al. The impact of bridging therapy prior to CAR-T cell therapies clincial outcomes of patients with relapsed refractory large B-cell lymphoma. Tct.fonfex.com. Feb 8, 2021; (accessed Nov 2023). Crossref
12. Gebo JET, Lau AF. Sterility testing for cellular therapies: what is the role of the clinical microbiology laboratory? J. Clin. Microbiol. 2020; 58(7), e01492–19. Crossref
13. Totten AH, Adams AJ, Halas HK, et al. Comparison of five commercial molecular assays for mycoplasma testing of cellular therapy products. J. Clin. Microbiol. 2023; 61(2), e0149822. Crossref
14. Biomerieux.com. With a complete portfolio of Quality Control solutions, bioMérieux ensures the safety of patients undergoing cell and gene therapies (accessed Nov 2023). Crossref
15. Biomerieux.com. Quality solutions to support manufacturing of cell and gene therapies (accessed Nov 2023). Crossref
16. United States Pharmacopeia. General Chapter, {63} Mycoplasma Tests. USP-NF. Rockville, MD: United States Pharmacopeia (2023). Crossref
17. European Pharmacopoeia Commission. European Pharmacopoeia 11th Edition 2022. Chapter 2.6.7 Mycoplasmas. Crossref
18. United States Pharmacopeia. General Chapter, {71} Sterility Tests. USP-NF. Rockville, MD: United States Pharmacopeia (2023). Crossref
19. European Pharmacopoeia Commission. European Pharmacopoeia 11th Edition 2022. Chapter 2.6.1 Sterility. Crossref
20. Hort S, Herbst L, Bäckel N, et al. Toward rapid, widely available autologous CAR-T cell therapy—artificial intelligence and automation enabling the smart manufacturing hospital. Front. Med. (Lausanne) 2022; 9, 913287. Crossref
21. Alliancerm.org. (accessed Nov 2023). Crossref
22. Yang J, He J, Zhang X, et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study. Blood Cancer J. 2022; 12(7), 104. Crossref
23. Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022; 21(1), 78. Crossref
24. Xin T, Cheng L, Zhou C, et al. In vivo induced CAR-T cell for the potential breakthrough to overcome the barriers of current CAR-T cell therapy. Front. Oncol. 2022; 12, 809754. Crossref
Affiliations
Narjes Armanet MD PhD
Senior Global Medical Advisor,
bioMérieux
Felix A Montero Julian PhD
Senior Scientific Director,
bioMérieux
Authorship & Conflict of Interest
Contributions: The named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & Copyright Information
Copyright: Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © bioMérieux. Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Jan 29, 2024; Revised manuscript received: Feb 15, 2024; Publication date: Feb 28, 2024.

