Cryopreservation: best practices for cell-based products
Cell & Gene Therapy Insights 2024; 10(1), 187
10.18609/cgti.2024.028
Published: 14 March 2024
Infographic
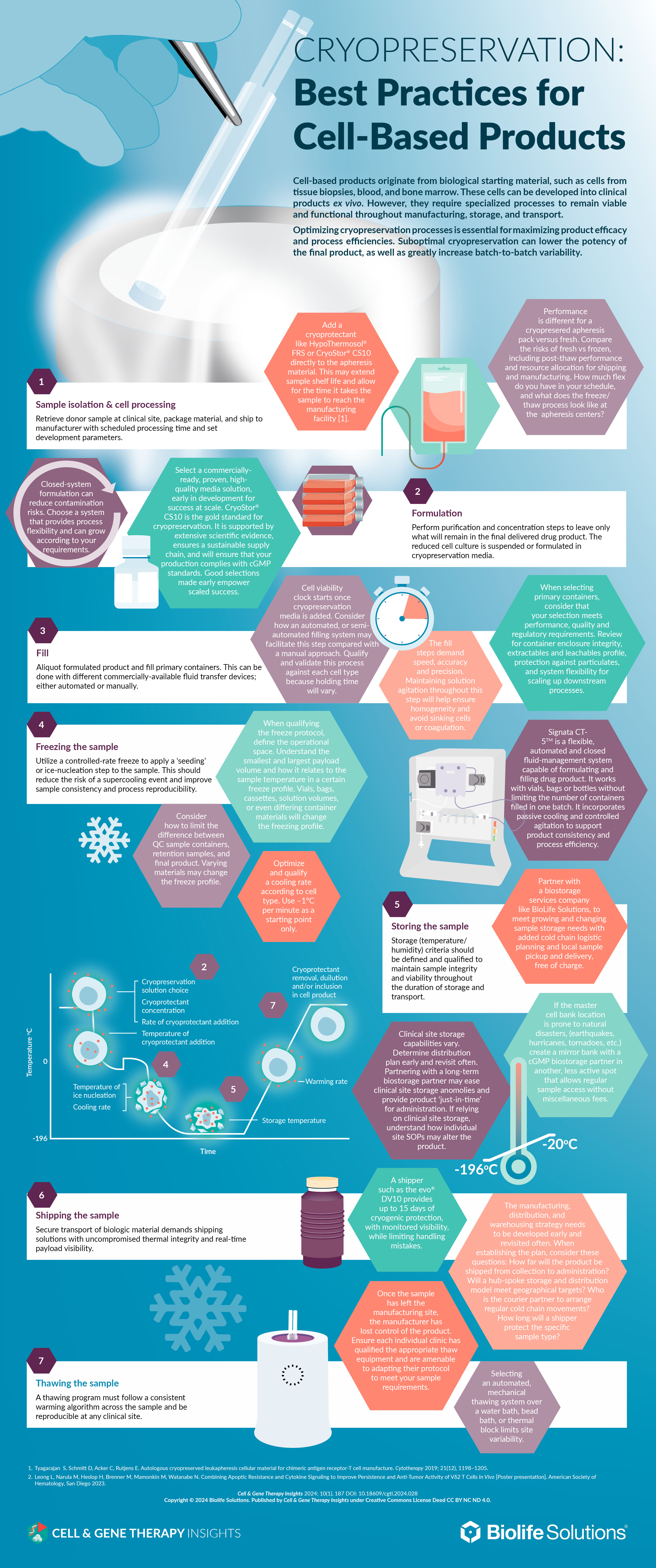
 | Cell-based products originate from biological starting material, such as cells from tissue biopsies, blood, and bone marrow. These cells can be developed into clinical products ex vivo. However, they require specialized processes to remain viable and functional throughout manufacturing, storage and transport. Optimizing cryopreservation processes is essential for maximizing product efficacy and process efficiencies. Suboptimal cryopreservation can lower the potency of the final product, as well as greatly increase batch-to-batch variability. |
 | You can download and print the infographic here Brought to you in partnership with: |

