Advancing AAV production with high-throughput screening and transcriptomics
Cell & Gene Therapy Insights 2024; 10(6), 821–840
DOI: 10.18609/CGTI.2024.095
AAV is a widely used vector for in vivo gene delivery that often requires considerable manufacturing capacity. However, current manufacturing techniques are inefficient, leading to high production costs and product impurities that could limit efficacy and risk patient safety. These factors severely limit the widespread use of AAV-mediated gene therapies for common and rare indications. To develop, manufacture, and commercialize AAV products more efficiently and cost effectively, the manufacturing process needs improvements. Herein, we transcend traditional strategies for developing DoE processes by sharing a new integrated approach that combines high-throughput screening and transcriptomics. This approach can yield proprietary datasets and insight into the mechanisms of AAV production. We also outline how discoveries and innovations in upstream production can be improved to amplify the product yield and quality over the next decade. These efforts will enable the field to fully realize the commercial and therapeutic promise of AAV gene therapies.
AAV-based gene therapy has emerged as a mainstay of modern medicine
AAVs are nonpathogenic dependoparvoviruses that contain a single-stranded DNA genome of approximately 4.7 kb [1,2]. Recombinant AAVs have gained popularity in the gene therapy field due to their well-established safety profile, broad and tunable tropism, nonpathogenic nature, and capability of achieving long-term expression in non-dividing cells with a single treatment. Also, with capsid engineering, the tropism of these vectors can be enhanced to increase the efficiency of transduction of the desired tissue type, limit the host immune response, and de-target from undesired tissues to improve safety [3,4]. As of 2024, AAV gene therapies have been administered to more than 3000 patients, and more than 700 programs are currently being developed (Figure 1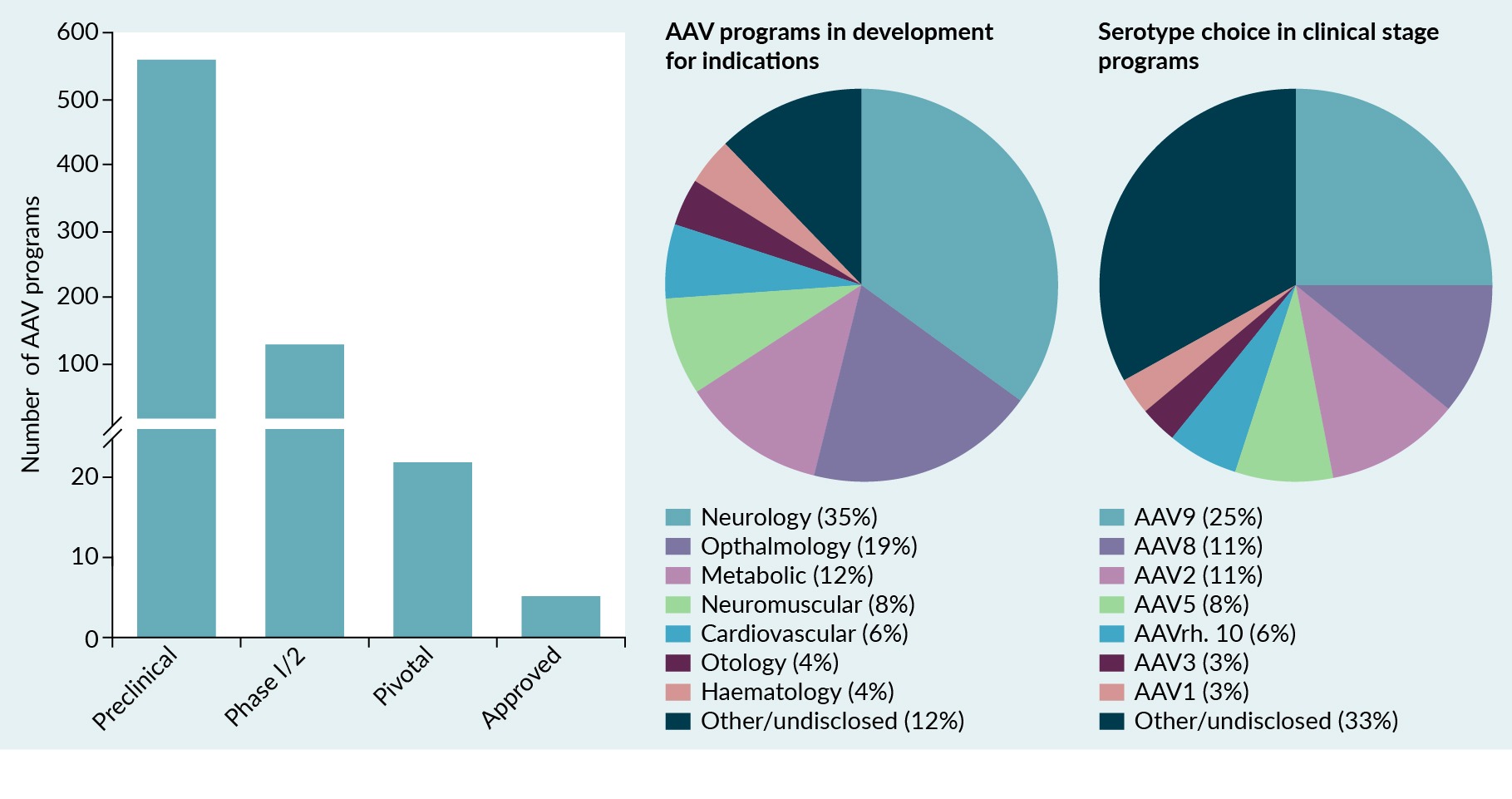 Landscape of AAV gene therapy programs.More than 700 AAV programs are being developed for a range of indications, including neurologic, ophthalmologic, and metabolic conditions. The most commonly used AAV serotypes used in clinical trials include AAV9, AAV8, AAV2, and AAV5.) [5–9]. These programs focus on a range of indications, including neurologic, ophthalmologic, metabolic, neuromuscular, and cardiovascular diseases. They also use a variety of AAV serotypes. The four most common AAV serotypes used in clinical trials and approved as gene therapies are AAV9, AAV8, AAV2, and AAV5 (Figure 1). As a gene-delivery vehicle, AAV is generally safe and well-tolerated at doses below 1 × 1014 vg/kg [5,10]. However, doses greater than 1 × 1014 vg/kg need more careful monitoring in patients to assess potential safety concerns in the context of the disease status [11].
Landscape of AAV gene therapy programs.More than 700 AAV programs are being developed for a range of indications, including neurologic, ophthalmologic, and metabolic conditions. The most commonly used AAV serotypes used in clinical trials include AAV9, AAV8, AAV2, and AAV5.) [5–9]. These programs focus on a range of indications, including neurologic, ophthalmologic, metabolic, neuromuscular, and cardiovascular diseases. They also use a variety of AAV serotypes. The four most common AAV serotypes used in clinical trials and approved as gene therapies are AAV9, AAV8, AAV2, and AAV5 (Figure 1). As a gene-delivery vehicle, AAV is generally safe and well-tolerated at doses below 1 × 1014 vg/kg [5,10]. However, doses greater than 1 × 1014 vg/kg need more careful monitoring in patients to assess potential safety concerns in the context of the disease status [11].
As of June 2024, 7 AAV gene therapy products are commercially available and approved by either the European Medicines Agency (EMA) or the US FDA (Table 1). In 2012, Glybera® became the first approved AAV gene therapy by the EMA for treating lipoprotein lipase deficiency. However, 5 years later, Glybera was withdrawn from the market due to commercial considerations [12]. Current approved AAV products include Luxturna® for Leber’s congenital amaurosis, a rare inherited retinal dystrophy; Zolgensma® for spinal muscular atrophy type 1; Upstaza™ for aromatic l-amino acid decarboxylase deficiency; Hemgenix® and Beqvez™ for hemophilia B; Roctavian™ for hemophilia A; and Elevidys® for Duchenne muscular dystrophy [4,13]. Several more therapies are in late-stage clinical trials, and the FDA predicts that 10–20 cell and gene therapy products may be approved by 2025 [14].
| Table 1. AAV-based gene therapy products that are commercially available and approved as of 2024. | ||||||
|---|---|---|---|---|---|---|
| AAV therapy (company) | Indication | Vector | Manufacturing platform | Approval | Dose | Price, million $ USD (2024) |
| Luxturna® (Roche) | Leber congenital amaurosis | AAV2 RPE65 | HEK293 | 2017 (FDA) 2019 (EMA) | 1.5 × 1011 vg/eye | 0.85 |
| Zolgensma® (Novartis) | Spinal muscular atrophy | AAV9 SMN1 | HEK293 | 2019 (FDA) 2020 (EMA) | 1.1 × 1014 vg/kg | 2.1 |
| Upstaza™ (PTC Therapeutics) | Aromatic l-amino acid decarboxylase deficiency | AAV2 DDC | HEK293 | 2022 (EMA) | 1.8 × 1011 vg | 3.7 |
| Hemgenix® (CLS Behring) | Hemophilia B | AAV5 F9 | Sf9 | 2022 (FDA) 2023 (EMA) | 2 × 1013 vg/kg | 3.5 |
| Roctavian™ (BioMarin) | Hemophilia A | AAV5 F8 | Sf9 | 2022 (EMA) 2023 (FDA) | 6 × 1013 vg/kg | 2.9 |
| Elevidys® (Sarepta Therapeutics) | Duchenne muscular dystrophy | AAVrh74 Micro-DMD | HEK293 | 2023 (FDA) | 1.33 × 1014 vg/kg | 3.2 |
| Beqvez™ (Pfizer) | Hemophilia B | AAVrh74 F9 | HEK293 | 2024 (FDA) | 5 × 1011 vg/kg | 3.5 |
| HEK293: Human embryonic kidney 293. | ||||||
Current AAV manufacturing practices
Existing AAV production platforms rely on cells for manufacturing. The most common AAV manufacturing platform uses mammalian cells, primarily human embryonic kidney 293 (HEK293) and its derivatives, and less commonly HeLa, baby hamster kidney (BHK), and Chinese hamster ovary (CHO) cells. In these platforms, genetic material needed for recombinant AAV (rAAV) production is delivered through plasmid transfection [15–20] or viral transduction [17,21,22]. Alternatively, non-mammalian systems rely on Spodoptera frugiperda (Sf9) insect cells and baculovirus transduction [23,24]. Most recently, a transient plant-based system was described for rAAV production [25]. Also, stable packaging and/or producer cell lines have been used for rAAV manufacturing after induction by chemicals or helper viruses [19,26–31]. So far, five of the commercial AAV products use HEK293 cells, and two use the Sf9-baculovirus system (Table 1).
Several factors can influence selection of the AAV manufacturing platform, including required yields, scalability, capsid serotype, and regulatory considerations. As of 2024, the top performing Sf9-baculovirus systems have produced higher volumetric yields than the HEK293 system [32,33]. However, there is no clear consensus on which platform is ideal for AAV production due to variations in quality and potency of the vector produced by each system. Some of these differences may be driven by capsid serotype, cargo, and platform [32,34–36]. Although most early-stage AAV programs do not explicitly disclose which manufacturing platform is used for viral production, available public information suggests that around 85% of current AAV manufacturing uses mammalian cells [37,38]. Therefore, in this commentary, we focus on the production system that uses transient transfection of HEK293 cells.
The process of manufacturing rAAV drug product is categorized into upstream (drug substance) and downstream (drug product) processes. The upstream process defines the vector yield, integrity, infectivity, and potency. The process also describes several crucial product-derived impurities, including, but not limited to, full/empty capsid ratios, residual plasmid and host-cell DNA packaging, and posttranslational capsid modifications. Thus, to improve the quality, potency, and yield of the final rAAV drug product, the upstream process must be optimized. During such optimization, typical refinement steps include producer cell–line selection, media formulation, plasmid sequence and ratio optimization, transfection reagent selection and optimization, cell expansion and scale-up, bioreactor process optimization and monitoring. The downstream process typically involves concentration of the producer cells, cell lysis, nuclease treatment, affinity or hydrophobic interaction chromatography for capture and purification, tangential flow filtration for concentration and rebuffering, ion exchange chromatography and/or ultracentrifugation polishing, and full capsid enrichment, formulation, fill, and finish (Figure 2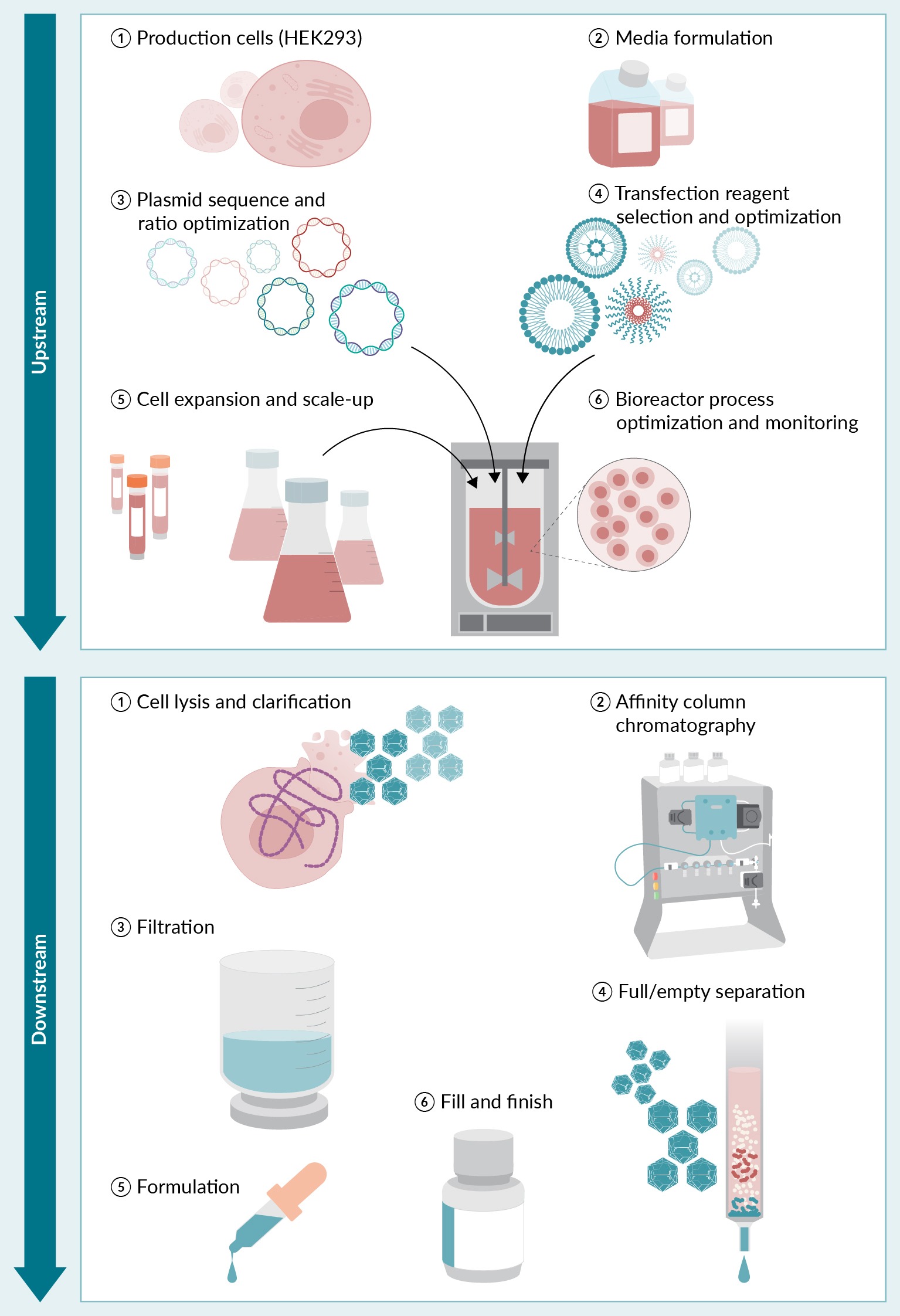 General overview of AAV production using plasmid transfection in mammalian cells.Upstream activities include cell-line selection, media formulation, plasmid sequence and ratio optimization, transfection reagent selection and optimization, cell expansion and scale-up, and bioreactor process optimization and monitoring. Downstream activities include concentration of raw materials, cell lysis, nuclease treatment, purification, polishing, formulation, fill, and finish.) [39,40].
General overview of AAV production using plasmid transfection in mammalian cells.Upstream activities include cell-line selection, media formulation, plasmid sequence and ratio optimization, transfection reagent selection and optimization, cell expansion and scale-up, and bioreactor process optimization and monitoring. Downstream activities include concentration of raw materials, cell lysis, nuclease treatment, purification, polishing, formulation, fill, and finish.) [39,40].
Manufacturing bottlenecks limit the widespread use of AAV gene therapies
Although rAAV-based medicines have shown long-term and robust safety and efficacy in the clinic, these treatments have prohibitive costs, particularly in the case of systemically delivered therapies [39]. For example, in 2019, Zolgensma became the most expensive medicine developed, with a price of US$2.1 million per patient for treating spinal muscular atrophy [41]. In 2022, Hemgenix and Upstaza surpassed Zolgensma as the most expensive drug, with a one-time price of US$3.5 million (Hemgenix) and US$3.7 million (Upstaza) per patient (Table 1) [42].
Another obstacle in slow adoption of rAAV gene therapies involves challenges with manufacturing. These challenges include non-scalable processes inherited from academic settings [43]; batch-to-batch variability [4]; poor quality due to low full-to-empty capsid ratios; packaging of undesired, potentially harmful DNA sequences [40,44]; capacity constraints; shortage of trained experts; and higher regulatory demands for manufacturing stringency [39,45]. Moreover, due to the relative novelty of AAV gene therapies, product developers have prioritized accelerating their medicines through clinical development and regulatory approval. This strategy often overlooks the opportunity and need to optimize manufacturing processes during the early stages of product development. These problems then persist after therapies are approved because product developers have little incentive to improve the established process that gained regulatory approval.
A move toward AAV therapies for more common indications
Fortunately, the demand for viral production has not yet been great enough to strain the system. The lower demand is because rAAV-based gene therapies have mainly targeted ultra-rare and rare monogenic diseases, such as Leber’s congenital amaurosis, spinal muscular atrophy, and hemophilia A and B. However, rAAV therapies are being explored for more common indications, such as Duchenne muscular dystrophy, that require a high systemic dose of vector [10]. These therapies are rapidly advancing through the clinic and will require a considerable amount of vector to keep up with patient demand [46]. Moreover, as rAAV-based gene therapies continue to build momentum, they will likely be developed to address more prevalent conditions. Indeed, preclinical programs are already targeting common diseases, such as diabetes [47], hypertension [48], Alzheimer’s disease [49,50], and heart failure [51–53].
Due to the growth of AAV gene therapies and their potential to treat highly prevalent conditions, the demand for viral production will rapidly exceed the capacity of manufacturers if processes are not improved (Figure 3![Volume of bioreactor needed to treat patient populations per year for either approved or pipeline AAV gene therapy programs per indication. The bioreactor capacity was calculated based on the current industry standard (average yield of 3 x 1014 vg/L and a 25% recovery). The estimated yearly number of patients included those in the USA, Canada, EU5, and Australia. The vector needed for systemic dosing was calculated based on the average weight of 75 kg for adult patients, 18 kg for 5-year-old patients receiving Elevidys, and 12 kg for 2-year-old patients receiving Zolgensma. The calculations also assume a market penetration or drug adoption of 25% for genetic heart failure (dilated cardiomyopathy [DCM] and hypertrophic cardiomyopathy [HCM]), 1% for general heart failure, 1% for metabolic diseases (diabetes), and 1% for neurodegenerative diseases (Alzheimer’s disease [AD], Huntington’s disease [HD], and frontotemporal dementia [FTD] combined). Volume of bioreactor needed to treat patient populations per year for either approved or pipeline AAV gene therapy programs per indication. The bioreactor capacity was calculated based on the current industry standard (average yield of 3 x 1014 vg/L and a 25% recovery). The estimated yearly number of patients included those in the USA, Canada, EU5, and Australia. The vector needed for systemic dosing was calculated based on the average weight of 75 kg for adult patients, 18 kg for 5-year-old patients receiving Elevidys, and 12 kg for 2-year-old patients receiving Zolgensma. The calculations also assume a market penetration or drug adoption of 25% for genetic heart failure (dilated cardiomyopathy [DCM] and hypertrophic cardiomyopathy [HCM]), 1% for general heart failure, 1% for metabolic diseases (diabetes), and 1% for neurodegenerative diseases (Alzheimer’s disease [AD], Huntington’s disease [HD], and frontotemporal dementia [FTD] combined).](https://cdn.insights.bio/uploads/Figure/C_ASND_401II_f3_130.jpg) AAV therapies moving toward common indications and bioreactor capacity to address pipeline therapies.Volume of bioreactor needed to treat patient populations per year for either approved or pipeline AAV gene therapy programs per indication. The bioreactor capacity was calculated based on the current industry standard (average yield of 3 x 1014 vg/L and a 25% recovery). The estimated yearly number of patients included those in the USA, Canada, EU5, and Australia. The vector needed for systemic dosing was calculated based on the average weight of 75 kg for adult patients, 18 kg for 5-year-old patients receiving Elevidys, and 12 kg for 2-year-old patients receiving Zolgensma. The calculations also assume a market penetration or drug adoption of 25% for genetic heart failure (dilated cardiomyopathy [DCM] and hypertrophic cardiomyopathy [HCM]), 1% for general heart failure, 1% for metabolic diseases (diabetes), and 1% for neurodegenerative diseases (Alzheimer’s disease [AD], Huntington’s disease [HD], and frontotemporal dementia [FTD] combined).). Currently, most systemically delivered AAV gene therapies require a total dose ranging from 1 × 1014 to 1 × 1016 vg per patient [8]. However, as of 2024 the yields of mammalian-based AAV range from 1 × 1013 to 4 × 1015 vg/L depending on the expression cassette, vector length, and capsid serotype [8,54,55]. Assuming an average yield of 3 × 1014 vg/L, and a 25% recovery rate after purification [54], a 200 L bioreactor yields enough material to treat 1 to 10 patients with a systemically delivered mid-to-high dose of AAV gene therapy.
AAV therapies moving toward common indications and bioreactor capacity to address pipeline therapies.Volume of bioreactor needed to treat patient populations per year for either approved or pipeline AAV gene therapy programs per indication. The bioreactor capacity was calculated based on the current industry standard (average yield of 3 x 1014 vg/L and a 25% recovery). The estimated yearly number of patients included those in the USA, Canada, EU5, and Australia. The vector needed for systemic dosing was calculated based on the average weight of 75 kg for adult patients, 18 kg for 5-year-old patients receiving Elevidys, and 12 kg for 2-year-old patients receiving Zolgensma. The calculations also assume a market penetration or drug adoption of 25% for genetic heart failure (dilated cardiomyopathy [DCM] and hypertrophic cardiomyopathy [HCM]), 1% for general heart failure, 1% for metabolic diseases (diabetes), and 1% for neurodegenerative diseases (Alzheimer’s disease [AD], Huntington’s disease [HD], and frontotemporal dementia [FTD] combined).). Currently, most systemically delivered AAV gene therapies require a total dose ranging from 1 × 1014 to 1 × 1016 vg per patient [8]. However, as of 2024 the yields of mammalian-based AAV range from 1 × 1013 to 4 × 1015 vg/L depending on the expression cassette, vector length, and capsid serotype [8,54,55]. Assuming an average yield of 3 × 1014 vg/L, and a 25% recovery rate after purification [54], a 200 L bioreactor yields enough material to treat 1 to 10 patients with a systemically delivered mid-to-high dose of AAV gene therapy.
Optimization challenges with AAV production
As of 2024, most AAV manufacturers have maximized AAV yields by primarily focusing on DoE studies in upstream processes (e.g., plasmid ratio refinement, transfection reagent optimization, and media feed strategies) [54,56,57]. Although DoE studies are needed to optimize an existing manufacturing platform, their effectiveness is constrained within the boundaries of current manufacturing paradigms. For example, mammalian cells have not evolved to function as AAV production factories. HEK293 cells were historically chosen for bioproduction due to their ease of culture in both adherent and suspension cultures, rapid growth, and high transfection efficiency [20,58]. As a result, current cell-based systems may not support ‘super physiologic’ production of rAAVs. Therefore, DoE studies alone may not be enough to markedly enhance AAV production.
Another challenge in rAAV production is partly driven by our limited biological understanding of cellular processes involved in rAAV replication and assembly [40,59]. For example, rAAV production requires timely expression of AAV proteins, helper virus, and cellular gene products at appropriate levels; precise assembly of capsid proteins; as well as replication of vector genomes and their packaging into preformed empty capsids [4]. One study estimates that approximately 75% of rAAV capsids are empty or partially formed [54]. Another study suggests that only a fraction (approximately 7%) of cells produce assembled rAAV capsids when using a triple-transfection method, despite a 60% transfection efficiency [60]. These inefficiencies lead to low-volume yields of rAAV production that are approximately 1000–4000-fold lower than that of monoclonal antibody production [61].
An integrated approach to advancing AAV production
We believe that innovative strategies in engineering cells and processes can disrupt upstream processes and complement conventional DoE studies. We also believe that these strategies can improve both the yield and quality of the starting material at a log scale to reduce the strain on downstream processing. Although downstream processes are crucial for the final AAV product, we propose that directing more research and resources to disruptive solutions upstream will lead to a higher titer and quality starting point that streamlines the overall AAV production process.
To this end, we developed an integrative strategy that uses a:
- Systems biology approach powered by high-throughput screening and transcriptomics [62,63]; and
- Rational design involving data-driven evolution of our proprietary split 2-plasmid platform (Figure 4
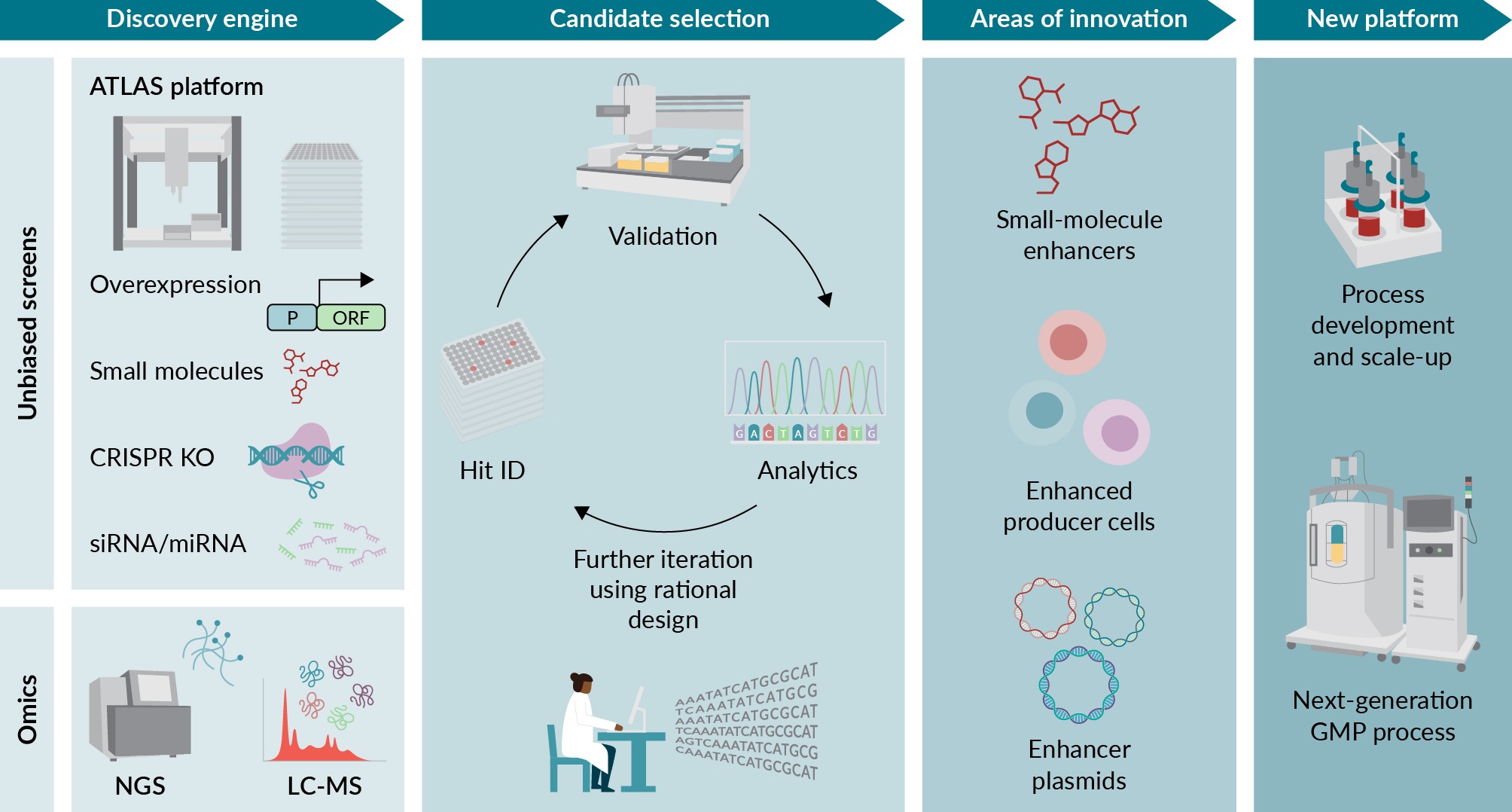 New commercial platform developed using an integrated approach to identify AAV enhancers.Arrayed Targeted Libraries for AAV Screening (ATLAS) is a high-throughput platform that can identify AAV enhancers in a 96-well format using gain-of-function or loss-of-function methods. Omic approaches can uncover mechanisms to identify key pathways during AAV production. The combination of these datasets is a rich source of proprietary data that can be mined and further refined with rational design to develop the next generation of AAV manufacturing. Areas of innovation could include discovering small-molecule enhancers or developing enhanced producer cell lines, plasmids, or sequences. CRISPR: Clustered regularly interspaced short palindromic repeats; ID: Identification; KO: Knockout; LC-MS: Liquid chromatography mass spectrometry; NGS: Next-generation sequencing; ORF: Open reading frame.) [64].
New commercial platform developed using an integrated approach to identify AAV enhancers.Arrayed Targeted Libraries for AAV Screening (ATLAS) is a high-throughput platform that can identify AAV enhancers in a 96-well format using gain-of-function or loss-of-function methods. Omic approaches can uncover mechanisms to identify key pathways during AAV production. The combination of these datasets is a rich source of proprietary data that can be mined and further refined with rational design to develop the next generation of AAV manufacturing. Areas of innovation could include discovering small-molecule enhancers or developing enhanced producer cell lines, plasmids, or sequences. CRISPR: Clustered regularly interspaced short palindromic repeats; ID: Identification; KO: Knockout; LC-MS: Liquid chromatography mass spectrometry; NGS: Next-generation sequencing; ORF: Open reading frame.) [64].
Specifically, we developed a 96-well platform to screen for AAV enhancers using Arrayed Targeted Libraries for AAV Screening (ATLAS) [65]. ATLAS can be used to induce gain-of-function, promote loss-of-function, and perturb pathways using microRNAs [66–69]. Although ATLAS can be used to test single perturbations, some perturbations may not ‘nudge’ the biological process or pathway enough to result in a detectable increase in AAV yield during the screening process. This result is particularly pronounced in complex biological systems that use multiple protein isoforms and multi-protein complexes. Thus, to better understand the mechanisms of AAV production and identify enhancers of AAV production, we propose to integrate complementary strategies: screening with transcriptomics and proteomics [16,62,63,70]. This synergistic approach accelerates the discovery of novel targets, enriches our hypothesis generation, and offers a robust framework for understanding the complex biology underlying AAV production (Figure 4).
Our goal is to use this integrative approach to develop a rich dataset that captures both common and cell-specific enhancers of AAV production. Ultimately, this dataset can be leveraged along with rational design strategies and incorporated into the next generation of AAV manufacturing processes. These next generation processes could include improving media formulations (e.g., using small-molecule enhancers), introducing enhancer plasmids to optimize plasmid sequences (e.g., expressing shRNA and miRNA cassettes, enhancer open reading frames, or modified viral elements), and modifying genetic elements of the producer cell line (e.g., knocking out genes or overexpressing mammalian or viral elements).
Case study: high-throughput screening and transcriptional analysis identify common pathways perturbed during AAV production
To determine if data generated from a high-throughput AAV screen have commonalities with transcriptomics data collected during AAV production, we analyzed two internal datasets. We identified differentially expressed transcripts during AAV9 production with RNA-sequencing (Figure 5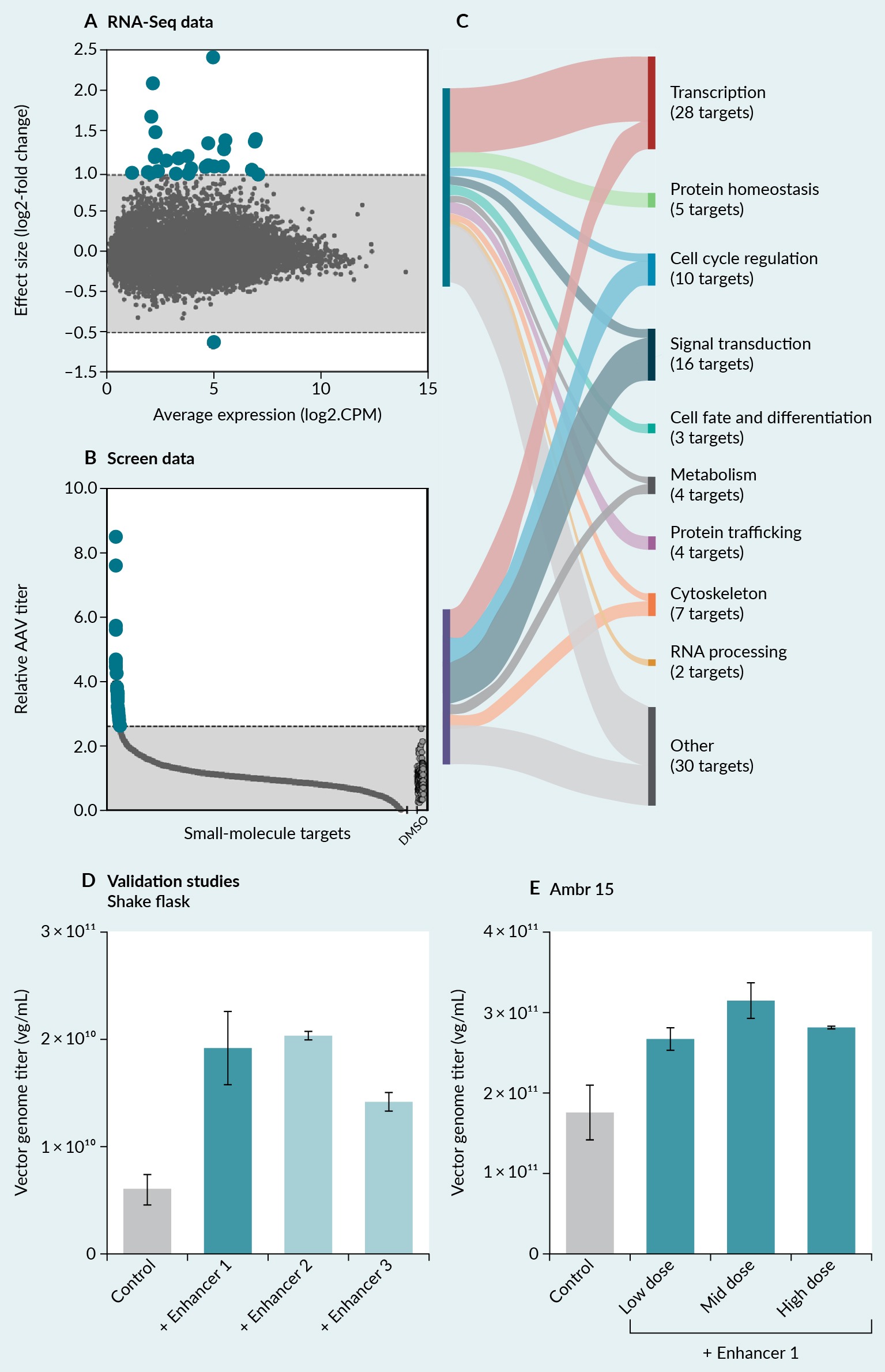 High-throughput screening and transcriptional analysis to identify putative pathways that enhance AAV production.(A) RNA-sequencing revealed differentially expressed transcripts during AAV9 production. (B) A high-throughput compound screen revealed small-molecule enhancers of AAV9 production. (C) Genes and pathways from two data sources were categorized into known biological pathways based on molecular function and gene ontology annotation. (D) The top three most-promising small-molecule enhancers improved AAV9 yields by 3.1-fold in suspension shake flasks. (E) Enhancer 1 was further advanced and improved AAV9 yields approximately 1.8-fold in an Ambr15 bioreactor system.A) [70] and enhancers of AAV9 production with a primary small-molecule screen using our ATLAS platform (Figure 5B) [68]. We then intersected the identified targets and categorized them into known biological pathways based on molecular function and gene ontology annotation. The top three shared pathways consisted of transcription, signal transduction, and cell-cycle regulation (Figure 5C). Some differentially altered pathways were identified with RNA-sequencing but not with the small-molecule screen (e.g., protein homeostasis, cell fate and differentiation, protein trafficking, RNA processing). This mismatch may be due to the composition of the small-molecule library and the limitation of the chemical probes targeting difficult drug targets and certain biological processes. Also, some pathways may be altered downstream because of AAV production, so modulation of those pathways would likely not affect AAV production. Next, we selected the top three most-promising small-molecule enhancers for studies in suspension shake flasks. All three enhancers yielded greater AAV9 yields compared to the control condition (Figure 5D). We validated the most-promising small-molecule enhancer in an Ambr15 bioreactor system at three doses (Figure 5E). Enhancer 1 shows a higher effect size on AAV9 yield in shake flakes (3.1-fold) compared to Amb15 (1.8-fold). The difference in relative fold enhancement may be attributed to a lower AAV production capacity observed in shake flasks compared to Amb15 bioreactors. This case study shows the feasibility of intersecting transcriptomics with high-throughput screening data to identify novel enhancers of AAV production and scale-up discoveries to develop more efficient processes.
High-throughput screening and transcriptional analysis to identify putative pathways that enhance AAV production.(A) RNA-sequencing revealed differentially expressed transcripts during AAV9 production. (B) A high-throughput compound screen revealed small-molecule enhancers of AAV9 production. (C) Genes and pathways from two data sources were categorized into known biological pathways based on molecular function and gene ontology annotation. (D) The top three most-promising small-molecule enhancers improved AAV9 yields by 3.1-fold in suspension shake flasks. (E) Enhancer 1 was further advanced and improved AAV9 yields approximately 1.8-fold in an Ambr15 bioreactor system.A) [70] and enhancers of AAV9 production with a primary small-molecule screen using our ATLAS platform (Figure 5B) [68]. We then intersected the identified targets and categorized them into known biological pathways based on molecular function and gene ontology annotation. The top three shared pathways consisted of transcription, signal transduction, and cell-cycle regulation (Figure 5C). Some differentially altered pathways were identified with RNA-sequencing but not with the small-molecule screen (e.g., protein homeostasis, cell fate and differentiation, protein trafficking, RNA processing). This mismatch may be due to the composition of the small-molecule library and the limitation of the chemical probes targeting difficult drug targets and certain biological processes. Also, some pathways may be altered downstream because of AAV production, so modulation of those pathways would likely not affect AAV production. Next, we selected the top three most-promising small-molecule enhancers for studies in suspension shake flasks. All three enhancers yielded greater AAV9 yields compared to the control condition (Figure 5D). We validated the most-promising small-molecule enhancer in an Ambr15 bioreactor system at three doses (Figure 5E). Enhancer 1 shows a higher effect size on AAV9 yield in shake flakes (3.1-fold) compared to Amb15 (1.8-fold). The difference in relative fold enhancement may be attributed to a lower AAV production capacity observed in shake flasks compared to Amb15 bioreactors. This case study shows the feasibility of intersecting transcriptomics with high-throughput screening data to identify novel enhancers of AAV production and scale-up discoveries to develop more efficient processes.
Sequential AAV platform development through a commercial and regulatory lens
In the case study, we showed an example of how early-stage research and development can lead to discoveries that enhance existing platforms for AAV manufacturing. However, to commercialize a new AAV manufacturing platform, the platform needs to meet both regulatory and commercial requirements. For example, new discoveries must be paired with a strong analytical toolkit to drive advances in AAV bioprocessing. We are systematically stacking discoveries—especially those with a combinatorial effect and target-independent biological pathways—and combining them with further upstream and downstream process innovation. With this approach, we aim to improve AAV yield and quality in a stepwise manner over the next few years (Figure 6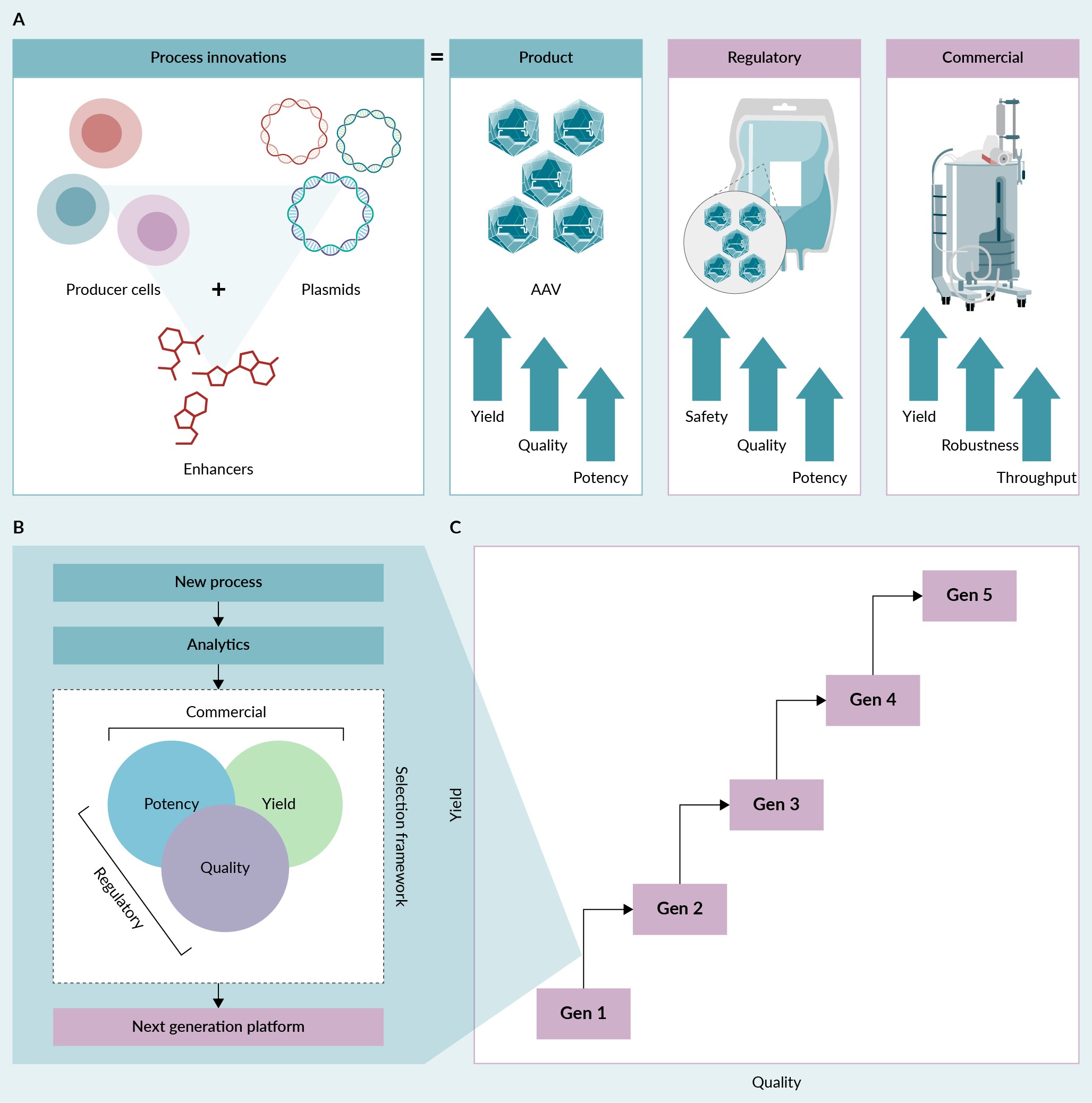 Process innovation and sequential AAV platform development.(A) Process innovations aim to enhance the yield, quality, and potency of AAV product to satisfy both regulatory and commercial standards. (B) New processes must be vetted using well-defined analytical methods to ensure a new manufacturing platform meets both commercial and regulatory requirements. (C) Advances in both yield and quality can be achieved by sequentially stacking discoveries and combining process innovations.).
Process innovation and sequential AAV platform development.(A) Process innovations aim to enhance the yield, quality, and potency of AAV product to satisfy both regulatory and commercial standards. (B) New processes must be vetted using well-defined analytical methods to ensure a new manufacturing platform meets both commercial and regulatory requirements. (C) Advances in both yield and quality can be achieved by sequentially stacking discoveries and combining process innovations.).
Importantly, the journey of innovation in bioprocessing must be navigated with caution to ensure that developments align with regulatory agencies and gene therapy developers. Without this alignment, regulatory agencies and gene therapy developers will be overwhelmed with rapid, groundbreaking process changes that could pose challenges. To avoid these challenges, developers need to adopt a balanced and gradual approach to introducing these new technologies. They also need to harmonize throughput and scalability by considering the intricacies of technology transfer throughout the scaling process, from microplates to shake flasks and, eventually, to small- and large-scale bioreactors. This harmony requires rigorous development, validation, and transfer of new technologies to ensure that each discovery is appropriately scaled and integrated into the manufacturing process. Through this balanced and systematic approach, we aim not only to innovate, but also to ensure the practical applicability and regulatory compliance of advances in producing AAV gene therapies. Our focus is to ensure continually improved product quality with the release and scale-up of each new-generation platform using our very broad analytic toolbox. With this approach, we aim to enable companies to move to larger scales or next-generation platforms within a product’s life cycle as the demand for vectors increases, without incurring significant chemistry, manufacturing, and controls risk in terms of lack of comparability.
Discussion and future directions
Manufacturing AAV-based therapies is complex and costly, which affect both regulatory (safety defined by vector quality and potency) and commercial (cost of goods and the number of deliverable doses per year, defined by vector yield and potency) aspects. The operational demands and high costs are a result of challenges with scalability, process robustness, and productivity maintenance that result in gene therapies often reaching millions of dollars per treatment.
The high cost of treatment has led to challenges in the commercialization of some AAV gene therapies, as proven by the cases of Glybera and, most recently, Roctavian. Glybera, the first gene therapy approved in Europe, was withdrawn in 2017 due to its high cost and lack of national reimbursement, even though it showed potential benefits for patients with lipoprotein lipase deficiency [12]. Roctavian was recently approved for hemophilia A but has faced slow uptake due to issues with reimbursement and market access, resulting in low numbers of treated patients and modest sales [71]. These cases highlight the acute pricing challenges with AAV gene therapies. The high upfront costs, combined with the need for long-term efficacy data and innovative payment models, create a complex commercial landscape. These experiences underscore the importance of establishing robust value propositions and ensuring broad access to make AAV gene therapies commercially viable.
The commercialization issues can be exacerbated by the rapid growth of bespoke and nascent AAV manufacturing processes in early-stage biotech companies. Although the transition from a bespoke AAV process to a clinical and, ultimately, commercial process has significant challenges, the transition offers substantial opportunities for innovation. For example, innovations in the manufacturing platform can enhance not only the yield and potency of the final drug product, but also the reproducibility, reducing batch failures, and ultimately shortening the development timeline and costs for sponsors. To address these issues, the FDA has encouraged early-stage companies to move their manufacturing processes to contract development and manufacturing organizations [43,46]. This shift aims to standardize procedures by drawing on strategies successfully implemented in the monoclonal antibody sector in the 1990s [72,73].
Despite similarities to monoclonal antibody production, AAV manufacturing poses unique challenges. For example, functional assembly of full AAV particles requires precise assembly of both capsid proteins and incorporation of nucleic acids [40,44,74]. Also, viral factors, including adenoviral helper elements and replicase proteins that are essential for replication and packaging, can lead to a DNA damage response and cell cycle arrest in producer cells [16]. Thus, to optimize AAV production, the expression and timing of these factors must be carefully calibrated.
To enhance manufacturing workflows, both processes and analytics must be standardized in collaboration with the FDA. As these advances are incorporated into manufacturing workflows, the cost of viral manufacturing will decline, and we may see a paradigm shift in the gene therapy business model. We also expect that the development of AAV gene therapies could expand beyond rare indications to also provide treatment options for common indications [46].
Looking ahead, we see the gene therapy industry moving toward progressively enhancing viral manufacturing platforms with several strategies. In this commentary, we described two strategies: high-throughput screening [75] and systems biology methods [16,63] to identify molecular targets that improve vector yield and quality. Some additional approaches that we did not discuss include optimizing vector sequences using machine learning [76,77], introducing synthetic DNA [78] to enhance vector potency, reducing plasmid-derived impurities, improving the safety and therapeutic index of AAV therapies, engineering viral proteins using mutagenesis screens [79], or using large language models [80] to enhance replication, packaging, and capsid assembly. Also, automating with robotics [73,81], using perfusion processes [15,82], and developing stable producer cell lines [19,26–31] can reduce the cost of goods and labor while also minimizing variation and batch failure during production campaigns. Finally, cell-free manufacturing may be among the next wave of innovations that could revolutionize viral manufacturing [83].
In addition to improving manufacturing methods and analytics, the safety and cost–effectiveness of AAV gene therapies must also be ensured by continuously optimizing product efficacy. This optimization is possible with both capsid selection and capsid engineering to improve the transduction of target cells, enhance the specificity of gene transfer, evade existing immune responses, and reduce de novo immune responses. The therapeutic expression cassette can also be optimized to improve safety and efficacy of the vector. This can be achieved through codon optimization and use of mRNA stabilizing elements to ensure optimal long-term expression. Additionally, using a tissue-specific promoter and miRNA de-targeting sequences can improve safety by restricting expression to the desired tissues.
Advanced drug developers also have the responsibility of maximizing the therapeutic index of potentially curative AAV medicines. This responsibility is crucial for providing safe and effective vectors to patients in urgent need. To achieve this goal, developers must learn from past experiences. For example, they need to address safety concerns that resulted in clinical holds due to poor vector quality [84] or that led to avoidable deaths due to immunotoxicities and liver dysfunction in patients treated with high doses of AAV gene therapies [11,85]. To provide safe and effective treatments, developers must meticulously conduct research and development to ensure that they learn from both past and present experiences.
Conclusion
In this commentary we describe current AAV manufacturing practices, and the next steps required to develop more efficient, scalable, and cost-effective manufacturing processes for AAV gene therapies. To develop the next-generation manufacturing platform, we use an integrated approach that combines high-throughput screening and RNA-sequencing. Our discovery approach provides a rich source of proprietary data that can be mined to identify candidate targets that enhance the quality and yield of AAV. After selecting a candidate, and using rational design for further improvements, our new manufacturing platform could incorporate enhanced small-molecules, producer cell lines, and plasmids or sequences.
By integrating novel technologic approaches with traditional manufacturing paradigms, we have outlined a promising path to greatly enhance the yield and quality of AAV vectors. This systems biology approach, complemented by the ATLAS platform for screening and transcriptomic analysis, accelerates the identification of crucial production enhancers and reveals the underlying biological mechanisms for efficient AAV production. As the demand for AAV therapies continues to grow, particularly for treating common diseases, the need for improved production processes becomes even more important. The continued innovation in bioprocessing, coupled with regulatory and commercial alignment, will ensure that these advanced manufacturing techniques meet clinical demands, comply with regulatory requirements, and remain economically viable. In the future, AAV manufacturing will likely incorporate diverse technologies (from machine learning to synthetic biology to automation), promising a new era of accessibility and effectiveness in gene therapy. This strategic shift is essential to surpass the current limitations and fulfill the therapeutic promise of AAV therapies for addressing a broad spectrum of diseases.
References
- Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014; 1(1), 427–451. Crossref
- Pupo A, Fernández A, Low SH, François A, Suárez-Amarán L, Samulski RJ. AAV vectors: the Rubik’s cube of human gene therapy. Mol. Ther. 2022; 30(12), 3515–3541. Crossref
- Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020; 21(4), 255–272. Crossref
- Wang JH, Gessler DJ, Zhan W, Gallagher TL, Gao G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal. Transduct. Target Ther. 2024; 9(1), 78. Crossref
- Kuzmin DA, Shutova MV, Johnston NR, et al. The clinical landscape for AAV gene therapies. Nat. Rev. Drug. Discov. 2021; 20(3), 173–174. Crossref
- Prasad R, Lugo T, Corwin S, Hanbury-Brown L, Boyle J. Under the Microscope State of the AAV Gene Therapy Landscape After Recent FDA AdCom on Safety. 2021 William Blair Equity Research. Crossref
- Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal. Transduct. Target Ther. 2021; 6(1), 53. Crossref
- Au HKE, Isalan M, Mielcarek M. Gene therapy advances: a meta-analysis of AAV usage in clinical settings. Front. Med (Lausanne) 2022; 8, 809118. Crossref
- Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019; 18(5), 358–378. Crossref
- Burdett T, Nuseibeh S. Changing trends in the development of AAV-based gene therapies: a meta-analysis of past and present therapies. Gene Ther. 2023; 30(3–4), 323–335. Crossref
- High-dose AAV gene therapy deaths. Nat. Biotechnol. 2020; 38(8), 910. Crossref
- Senior M. After Glybera’s withdrawal, what’s next for gene therapy? Nat Biotechnol. 2017; 35(6), 491–492. Crossref
- FDA. https://www.fda.gov. Crossref
- Gottlieb S. Statement from FDA Commissioner Scott Gottlieb, MD and Peter Marks, MD, PhD, Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. FDA Jan 5, 2020. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics. Crossref
- Grieger JC, Soltys SM, Samulski RJ. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol. Ther. 2016; 24(2), 287–297. Crossref
- Chung CH, Murphy CM, Wingate VP, et al. Production of rAAV by plasmid transfection induces antiviral and inflammatory responses in suspension HEK293 cells. Mol. Ther. Methods Clin. Dev. 2023; 28, 272–283. Crossref
- Nagy A, Chakrabarti L, Kurasawa J, et al. Engineered CHO cells as a novel AAV production platform for gene therapy delivery. Sci. Rep. 2023; 13(1), 19210. Crossref
- Chadeuf G, Favre D, Tessier J, et al. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirus-induced amplification of the integrated rep-cap genome. J. Gene Med. 2000; 2(4), 260–268. Crossref
- Su W, Seymour LW, Cawood R. AAV production in stable packaging cells requires expression of adenovirus 22/33K protein to allow episomal amplification of integrated rep/cap genes. Sci. Rep. 2023; 13(1), 21670. Crossref
- Tan E, Chin CSH, Lim ZFS, Ng SK. HEK293 cell line as a platform to produce recombinant proteins and viral vectors. Front. Bioeng. Biotechnol. 2021; 9, 796991. Crossref
- Su W, Patrício MI, Duffy MR, Krakowiak JM, Seymour LW, Cawood R. Self-attenuating adenovirus enables production of recombinant adeno-associated virus for high manufacturing yield without contamination. Nat. Commun. 2022; 13(1), 1182. Crossref
- Thomas DL, Wang L, Niamke J, et al. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene Ther. 2009; 20(8), 861–870. Crossref
- Aslanidi G, Lamb K, Zolotukhin S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc. Natl. Acad. Sci. USA 2009; 106(13), 5059–5064. Crossref
- Marwidi Y, Nguyen HB, Santos D, et al. A robust and flexible baculovirus-insect cell system for AAV vector production with improved yield, capsid ratios and potency. Mol. Ther. Methods Clin. Dev. 2024; 32(2), 101228. Crossref
- Mathur PD, Xie Q, Wong K, et al. Production of different AAV serotypes using a novel plant-based viral vector manufacturing platform (ASGCT abstract 25). Mol. Ther. 2024; 32, 4S1. Crossref
- Merten OW. Development of stable packaging and producer cell lines for the production of AAV vectors. Microorganisms 2024; 12(2), 384. Crossref
- Lee Z, Lu M, Irfanullah E, Soukup M, Hu WS. Construction of an rAAV producer cell line through synthetic biology. ACS Synth. Biol. 2022; 11(10), 3285–3295. Crossref
- Jalšić L, Lytvyn V, Elahi SM, et al. Inducible HEK293 AAV packaging cell lines expressing Rep proteins. Mol. Ther. Methods Clin. Dev. 2023; 30, 259–275. Crossref
- Han J, Tam K, Tam C, Hollis RP, Kohn DB. Improved lentiviral vector titers from a multi-gene knockout packaging line. Mol. Ther. Oncolytics. 2021; 23, 582–592. Crossref
- Nitschke P. ELEVECTA®—the next level in gene therapy AAV production. BioPharm Intl. 2020. Crossref
- Challener C. Proprietary cell-line development for high-titer AAV manufacturing. BioPharm Intl. 2024; 37, 10–15. Crossref
- Liu S, Li J, Peraramelli S, et al. Systematic comparison of rAAV vectors manufactured using large-scale suspension cultures of Sf9 and HEK293 cells. Mol. Ther. 2024; 32(1), 74–83. Crossref
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene. Ther. 2002; 13(16), 1935–1943. Crossref
- Rumachik NG, Malaker SA, Poweleit N, et al. Methods matter: standard production platforms for recombinant AAV produce chemically and functionally distinct vectors. Mol. Ther. Methods Clin. Dev. 2020; 18, 98–118. Crossref
- Kondratov O, Marsic D, Crosson SM, et al. Direct head-to-head evaluation of recombinant adeno-associated viral vectors manufactured in human versus insect cells. Mol. Ther. 2017; 25(12), 2661–2675. Crossref
- Rao R, Farraha M, Logan GJ, et al. Performance of cardiotropic rAAV vectors is dependent on production method. Viruses 2022; 14(8), 1623. Crossref
- ASGCT. 2023 ASGCT Abstract Book. Mol. Ther. 2023; 31, 4S1. Crossref
- ASGCT. 2024 ASGCT Abstract Book. Mol. Ther. 2024; 32, 4S1. Crossref
- Smith J, Grieger J, Samulski RJ. Overcoming bottlenecks in AAV manufacturing for gene therapy. Cell Gene Ther. Ins. 2018; 4(8), 815–825. Crossref
- Srivastava A, Mallela KMG, Deorkar N, Brophy G. Manufacturing challenges and rational formulation development for AAV viral vectors. J. Pharm. Sci. 2021; 110(7), 2609–2624. Crossref
- Feuerstein A. At $2.1 million, newly approved Novartis gene therapy will be world’s most expensive drug. STAT 2019. https://www.statnews.com/2019/05/24/hold-novartis-zolgensma-approval. Crossref
- Naddaf M. Researchers welcome $3.5-million haemophilia gene therapy—but questions remain. Nature 2022; 612(7940), 388–389. Crossref
- Marks P. Enhancing gene therapy regulatory interactions. Expert Opin. Biol. Ther. 2022; 22(9), 1073–1074. Crossref
- Bennett A, Mietzsch M, Agbandje-McKenna M. Understanding capsid assembly and genome packaging for adeno-associated viruses. Future Virol. 2017; 12(6), 283–297. Crossref
- Clément N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016; 3, 16002. Crossref
- Marks P, Witten C. Toward a new framework for the development of individualized therapies. Gene Ther. 2021; 28(10–11), 615–617. Crossref
- Jackson CB, Richard AS, Ojha A, et al. AAV vectors engineered to target insulin receptor greatly enhance intramuscular gene delivery. Mol. Ther. Methods Clin. Dev. 2020; 19, 496–506. Crossref
- Phillips MI, Mohuczy-Dominiak D, Coffey M, et al. Prolonged reduction of high blood pressure with an in vivo, nonpathogenic, adeno-associated viral vector delivery of AT1-R mRNA antisense. Hypertension 1997; 29(1 Pt 2), 374–380. Crossref
- Rosenberg JB, Kaplitt MG, De BP, et al. AAVrh.10-mediated APOE2 central nervous system gene therapy for APOE4-associated Alzheimer’s disease. Hum. Gene Ther. Clin. Dev. 2018; 29(1), 24–47. Crossref
- Yang Y, Seok MJ, Kim YE, et al. Adeno-associated virus (AAV) 9-mediated gene delivery of Nurr1 and Foxa2 ameliorates symptoms and pathologies of Alzheimer disease model mice by suppressing neuro-inflammation and glial pathology. Psychiatry 2023; 28(12), 5359–5374. Crossref
- Wu I, Zeng A, Greer-Short A, et al. AAV9:PKP2 improves heart function and survival in a Pkp2-deficient mouse model of arrhythmogenic right ventricular cardiomyopathy. Commun. Med. (Lond). 2024; 4(1), 38. Crossref
- Manso AM, Hashem SI, Nelson BC, et al. Systemic AAV9.LAMP2B injection reverses metabolic and physiologic multiorgan dysfunction in a murine model of Danon disease. Sci. Transl. Med. 2020; 12(535), eaax1744. Crossref
- Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ. Res. 2012; 110(5), 777–793. Crossref
- Coplan L, Zhang Z, Ragone N, et al. High-yield recombinant adeno-associated viral vector production by multivariate optimization of bioprocess and transfection conditions. Biotechnol. Prog. 2024; 40(3), e3445. Crossref
- Xue W, Fulco C, Sha S, et al. Adeno-associated virus perfusion enhanced expression: a commercially scalable, high titer, high quality producer cell line process. Mol. Ther. Methods Clin. Dev. 2024; 32(2), 101266. Crossref
- Zhao H, Lee KJ, Daris M, et al. Creation of a high-yield AAV vector production platform in suspension cells using a design-of-experiment approach. Mol. Ther. Methods Clin. Dev. 2020; 18, 312–320. Crossref
- Kimura T, Ferran B, Tsukahara Y, et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci. Rep. 2019; 9(1), 13601. Crossref
- Dumont J, Euwart D, Mei B, Estes S, Kshirsagar R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit. Rev. Biotechnol. 2016; 36(6), 1110–1122. Crossref
- Nguyen TNT, Sha S, Hong MS, et al. Mechanistic model for production of recombinant adeno-associated virus via triple transfection of HEK293 cells. Mol. Ther. Methods Clin. Dev. 2021; 21, 642–655. Crossref
- Dash S, Sharon DM, Mullick A, Kamen AA. Only a small fraction of cells produce assembled capsids during transfection-based manufacturing of adeno-associated virus vectors. Biotechnol. Bioeng. 2022; 119(6), 1685–1690. Crossref
- Wright JF. AAV vector production: troublesome host innate responses in another setting. Mol. Ther. Methods Clin. Dev. 2023; 28, 412–413. Crossref
- Samoudi M, Masson HO, Kuo CC, Robinson CM, Lewis NE. From omics to cellular mechanisms in mammalian cell factory development. Curr. Opin. Chem. Eng. 2021; 32, 100688. Crossref
- Strasser L, Boi S, Guapo F, et al. Proteomic landscape of adeno-associated virus (AAV)-producing HEK293 cells. Int. J. Mol. Sci. 2021; 22(21), 11499. Crossref
- Hörer M, Sonntag F, Kober R. Plasmid system. EP3722434B1. 2019. Crossref
- Reid CA, Grafton F, Leveque-Eichhorn L, et al. ATLAS: A high-throughput gain and loss-of-function screening platform for optimizing AAV production (ASGCT abstract 1616). Mol. Ther. 2023; 31, 4S1. Crossref
- Wang C, Sheth M, Ispaso F, et al. A genome-wide high-throughput gain-of-function screen identifies novel targets for improved AAV9 production (ASGCT abstract 1505) Mol. Ther. 2024; 32, 4S1. Crossref
- Grafton F, Feitzinger R, Fisher K, Leveque-Eichhorn L, Reid CA, Mandegar MA. Targeted CRISPR/Cas9 screen identifies superior HEK293 cell lines for AAV9 production (ASGCT abstract 1334). Mol. Ther. 2023; 31, 4S1. Crossref
- Grafton F, Feitzinger R, Fisher K, Leveque-Eichhorn L, Reid CA, Mandegar MA. A high-throughput small molecule screen identifies targets that increase AAV9 production in suspension HEK293 cells (ASGCT abstract 921). Mol. Ther. 2023; 31, 4S1. Crossref
- Finkbeiner B, Burkhart M, Reichl S, et al. Whole genome high-throughput screen identified microRNAs enhancing rAAV production (ASGCT abstract 1058). Mol. Ther. 2024; 32–4S1. Crossref
- Tworig J, Grafton F, Hörer M, Reid CA, Mandegar MA. Transcriptomic analysis identifies differential expression patterns in cellular stress response, signal transduction, and extracellular matrix proteins during AAV production (ASGCT abstract 1063). Mol. Ther. 2024; 32, 4S1. Crossref
- Becker Z. With sales still stagnant, BioMarin adds divestiture to list of options for gene therapy. Roctavian Fiercepharma 2024. Crossref
- Jiang Z, Dalby PA. Challenges in scaling up AAV-based gene therapy manufacturing. Trends Biotechnol. 2023; 41(10), 1268–1281. Crossref
- Mirasol F. Gleaning scale-up lessons from mAbs for viral vectors. Pharm. Tech. 2023; 47, 20–21. Crossref
- Onishi T, Nonaka M, Maruno T, et al. Enhancement of recombinant adeno-associated virus activity by improved stoichiometry and homogeneity of capsid protein assembly. Mol. Ther. Methods Clin. Dev. 2023; 31, 101142. Crossref
- Barnes CR, Lee H, Ojala DS, Lewis KK, Limsirichai P, Schaffer DV. Genome-wide activation screens to increase adeno-associated virus production. Mol. Ther. Nucleic Acids 2021; 26, 94–103. Crossref
- Nipko J. Developing machine learning powered solutions for cell and gene therapy candidate validation form. Bio White Paper 2023. Crossref
- Ketz N. Leveraging model interpretability methods to predict gene therapy manufacturing failures form. Bio White Paper 2023. Crossref
- Karbowniczek K, Extance J, Milsom S, et al. Doggybone™ DNA: an advanced platform for AAV production. Cell Gene Ther. Ins. 2017; 3(9), 731–738. Crossref
- Jain NK, Ogden PJ, Church GM. Comprehensive mutagenesis maps the effect of all single-codon mutations in the AAV2 rep gene on AAV production. Elife 2024; 12:RP87730. Crossref
- Madani A, Krause B, Greene ER, et al. Large language models generate functional protein sequences across diverse families. Nat. Biotechnol. 2023; 41(8), 1099–1106. Crossref
- Tristan CA, Ormanoglu P, Slamecka J, et al. Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells. Stem Cell Reports 2021; 16(12), 3076–3092. Crossref
- Mendes JP, Fernandes B, Pineda E, et al. AAV process intensification by perfusion bioreaction and integrated clarification. Front. Bioeng. Biotechnol. 2022; 10, 1020174. Crossref
- Vilkhovoy M, Adhikari A, Vadhin S, Varner JD. The evolution of cell free biomanufacturing. Processes 2020; 8, 675. Crossref
- Solid Biosciences, Inc. Solid Biosciences announces FDA lifts clinical hold on IGNITE DMD clinical trial. Solid Biosciences 2020. Crossref
- Duan D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol. Ther. 2023; 31(11), 3123–3126. Crossref
Affiliations
Christopher A Reid PhD
Ascend Advanced Therapies CA Inc,
Alameda, CA, USA
Markus Hörer PhD
Ascend Advanced Therapies GmbH,
Planegg, Germany
Mohammad A Mandegar DPhil
Ascend Advanced Therapies CA Inc,
Alameda, CA, USA
Corresponding author
mandegar@ascend-gctx.com
Authorship & Conflict of Interest
Contributions: The named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: The authors would like to thank Crystal Herron of Redwood Ink, LLC for editing the manuscript, and the members of the research team at Ascend Advanced Therapies for helpful comments on this commentary.
Disclosure and potential conflicts of interest: The authors are employees of Ascend Advanced Therapies and have stock holdings in the company.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & Copyright Information
Copyright: Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2024 Ascend Advanced Therapies. Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Jun 10, 2024; Revised manuscript received: Jul 3, 2024; Publication date: Jul 23, 2024.

