Utilization of Recombinant Albumins in the Expansion of Human T Lymphocytes
Cell Gene Therapy Insights 2018; 4(4), 231-239.
10.18609/cgti.2018.024
As novel cellular therapeutics continue to demonstrate tremendous progress in the clinic, improvements in the understanding of cell culture media components and expansion media design must continue in parallel to achieve better manufacturing process definition and control. One such critical component, human serum albumin, has been demonstrated to promote growth and viability of mammalian cells when cultured ex vivo. Several serum free cell culture media products currently marketed incorporate albumin purified from human serum. Although these media formulations, known as xeno-free, have demonstrated satisfactory expansion capability of phenotypically correct cells, there are potential safety, supply, and reproducibility issues associated with using components isolated from serum. InVitria leverages an animal component free protein expression system to produce recombinant proteins, including human serum albumin, at large scale. The recombinant human serum albumin produced in a nonmammalian-based expression technology, known as Cellastim S®, exhibited exceptional ability to support expansion of T Lymphocytes sourced from peripheral blood of healthy donors in comparison to native sourced albumins from both human and bovine serum as well as other recombinant albumins produced in yeast. Further, Cellastim S® resembled serum-derived albumin albeit more consistent profile as determined by reverse phase HPLC and mass spectrometry. Taken together, Cellastim S® is an attractive recombinant alternative for serum-derived albumin in T Lymphocyte applications.
Submitted for peer review: Mar 8 2018 Published: May 14 2018
As cell therapies continue to gain tremendous progress and translate to promising innovative treatments for those battling severe diseases, improvements in the understanding of cell culture media components and expansion media design that support these novel therapies must continue in parallel to achieve a higher degree of manufacturing process definition and control. One such critical component is human serum albumin. This ~66 kD serum protein is one of the most abundant components present in the plasma of all vertebrates [1]. Albumin consists of a single-chain nonglycosylated polypeptide that forms the primary structure and folds into mostly α-helices with an overall structure that resembles a heart [2]. Cumulatively, serum albumin consists of 9 double loops that span 3 homologous domains dubbed Domain I-III. Each of these 3 domains consists of 2 long loops (subdomain A) and 1 short loop (subdomain B) [2].
Human serum albumin has evolved to have primary roles in many different important biological processes. Albumin is responsible for the majority of colloidal osmotic pressure in the circulatory system as a direct result of the high concentration in plasma (35-50 mg/mL) [3]. Further, albumin can bind a multitude of molecules including ions, fatty acids, and steroids and can deliver these compounds to cells [1,4]. Albumin in itself is also a potent antioxidant, waste carrier, and reactive oxygen/nitrogen species scavenger [3].
The broad functional aspects of albumin enables the significant enhancement of cell growth and viability of mammalian cells cultured ex vivo. The high concentration of serum albumin in human plasma has enabled the extraction and purification of this protein for use in mammalian cell culture. Several serum-free cell culture media products that incorporate human serum albumin are suitable for both manufacturing and research and development are currently available on the market. These media products, classified as being xeno-free since they incorporate human serum-derived components, demonstrate excellent ability in supporting cellular proliferation ex vivo and are more extensively defined than their full serum-containing media counterparts [5]. However, these native albumin-containing serum-free media still possess the inherent risk of variability that comes with any serum-derived component. Given the central functional role of albumin both in vivo and in vitro combined with the high concentrations in cell culture media, inconsistencies can greatly affect the overall media performance.
InVitria, a division of Ventria Bioscience, leverages an animal component free protein expression system to produce recombinant proteins at large scale that are critical for cell culture applications, including human serum albumin [6]. Further, downstream purification processes have enabled the refinement of recombinant human albumin products that demonstrate differential functional profiles in mammalian cell culture. One particular recombinant albumin, known as Cellastim S®, has exhibited exceptional ability to support cell growth in multiple cell types in vitro [7]. Here, the performance of Cellastim S® is demonstrated in expanding human T Lymphocytes sourced from peripheral blood of healthy donors in comparison to native sourced albumins from both human and bovine serum as well as other recombinant albumins. In addition, the biochemical characteristics of Cellastim S® are compared to serum-derived albumins.
Materials & Methods
Albumins
Yeast-derived recombinant albumins Recombumin Alpha®, Prime®, and AlbIX® products were purchased from Albumedix. Serum-derived albumins were sourced from Baxter and Grifols while bovine albumin was sourced from Sigma Aldrich and AlbuMAX® from Life Technologies. Cellastim S® was sourced from InVitria. Cellastim S® was reconstituted according to the guidelines for use and used as a fresh solution. All liquid albumins were not manipulated in any way prior to the experiments and stored according to the manufacturer’s instructions.
Cells
Peripheral blood from healthy donors were obtained from a commercial source (ZenBio, Research Triangle Park, NC). All blood samples were processed at a maximum of 48 hours post collection. To isolate T Lymphocytes, PBMC were isolated from whole blood using a Lymphoprep gradient with 1.077±0.001 g/mL density (StemCell Technologies, Vancouver, BC) and CD3+ cells were subsequently isolated via negative selection according to the manufacturer’s instructions (Stem Cell Technologies, Vancouver, BC). CD3+ cells were washed (3x) in basal RPMI (Life Technologies, Grand Island, NY) to remove all traces of donor serum prior to cell seeding.
Growth Comparison
To compare the performance of different albumins in expanding T Lymphocytes, a proprietary animal component free, chemically defined media (OptiPEAK T Lymphocyte, InVitria, Junction City, KS) was utilized. This media, which contains rhInsulin and rhTransferrin, was supplemented with 700 mg/L of each albumin in addition to 10 ng/mL rhIL-2 (Peprotech, Rocky Hill, NJ) and 0.5x Gentamicin/Amphotericin (Life Technologies, Grand Island, NY) to generate 9 different medias. T Lymphocytes were seeded into each medium at an initial cell density of 0.2-0.5e6 cells/mL in the presence of anti CD3/CD28 Activating Dynabeads (Life Technologies, Grand Island, NY) at a ratio of 1:1. Cells were expanded for 7 days with the media volume being doubled every other day. At day 7, viable cells were counted via fluorescent viability stain (Viacount, Millipore Sigma, Burlington, MA) using a flow cytometer (Millipore EasyCyte, Burlington, MA) and a fold increase was calculated based on the number of cells initially seeded. A total of 3 donors were analyzed and the difference of average fold expansions compared to 0 albumin were determined via a One Way ANOVA. *** p ≥ 0.001.
RP-UPLC & mass spectrometry
Reverse-phase analytical chromatography (RP-UPLC) was carried out on Agilent Infinity II 1290 UPLC system (Agilent, Santa Clara, CA) with UV-VIS detection using Waters AQUITY UPLC Protein BEH C4, 300A, 1.7um, 2.1x150mm column. Intact mass analysis was done by liquid chromatography-mass spectrometry (LC-MS) on a compact QqTOF mass spectrometer (Bruker, Billerica, MA) with Dionex UltiMate 3000 RSLC HPLC (Thermo Fisher, Waltham, MA) using Acclaim PepMap C300 C4 1x150mm column (Thermo Fisher, Waltham, MA). Acquisition and processing was done by oTOF Control v3.4 and DataAnalysis v4.2.
Results
Ability of different albumins to expand human T Lymphocytes from peripheral blood
Isolated T Lymphocytes fail to significantly expand in the absence of albumin in the chemically defined media, indicating the central role this protein has in the expansion of this cell type in vitro (Figure 1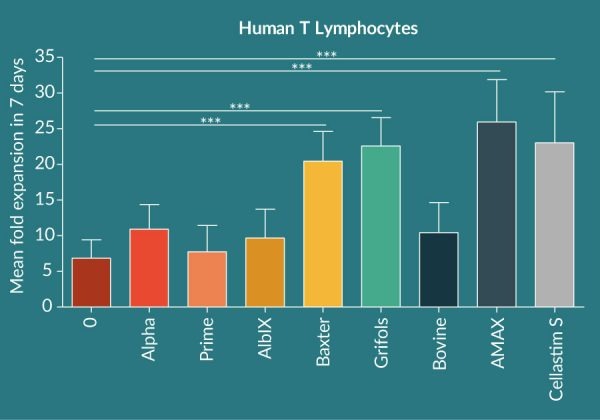
Consistency of Cellastim S in expanding human T Lymphocytes from peripheral blood
To assess the overall lot to lot variability of Cellastim S® four lots of Cellastim S® were analyzed for the ability to expand human T Lymphocytes in vitro in the OptiPEAK T Lymphocyte ACF SFM and compared to popular xeno-free serum-free media currently marketed as well as RPMI + 10% FBS. A total of four independent donors were analyzed and compared. Cellastim S® lots exhibited on average an equivalent ability to significantly expand T Lymphocytes (Figure 2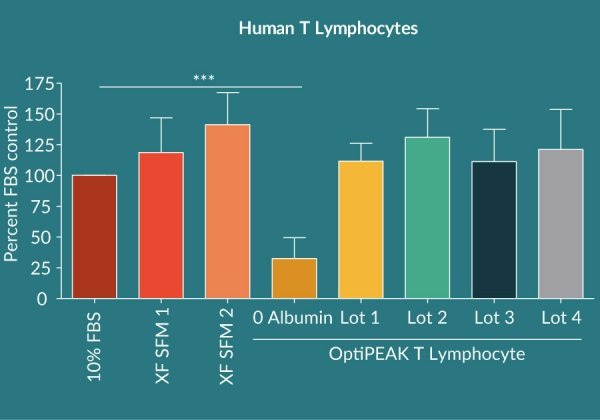
Analytical attributes of Cellastim S compared with human serum albumin via LC-MS and RP-UPLC
To further characterize the serum-derived albumins in addition to the multiple lots of Cellastim S®, reverse RP-UPLC and intact LC-MS was employed. Two methods revealed the presence of two major species in albumin: unmodified albumin species with unmodified Cys-34 and modified albumin species with cysteinylated Cys-34. Unmodified albumin species in the Baxter and Grifols albumin products has a mass of 66,438 Da on deconvoluted mass spectrum and migrates in the second major peak on RP-UPLC with retention time around 20.5min (Figure 3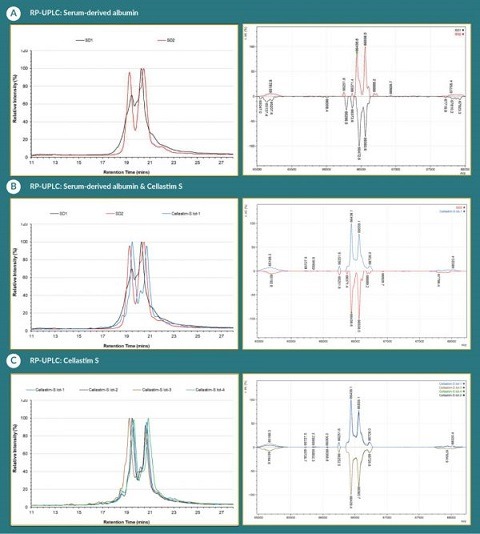
Discussion
Cellular therapeutics will continue to prove their utility in the clinic against devastating human diseases. Cases of success are only expected to bolster the confidence of the industry and encourage further clinical progress and investment in developmental pipelines. However, as this space continues to see unrivaled growth in the coming years, the expected pains that come with that growth are already becoming obvious. Namely, concerns in the availability of reliable supply of both human serum and human serum-albumin with consistent and expected performance are a notable topic amongst different process development groups.
In terms of security of supply, the current global demand for human serum albumin is approximately 663,000 kg and is increasing due to the application of human serum albumin in a multitude of therapeutic and emerging biotech developments [8,9]. Human serum albumin is typically sourced from plasmapheresis, although both time-expired blood and placental material have also been utilized [8,10]. Other plasma-derived components, such as Alpha-1 antitrypsin, immunoglobulin fractions, and clotting factors, are also utilized and thus reduces the amounts of available donor plasma for albumin purification. Overall, it is projected that 42 million liters of plasma will be recoverable in 2018 from the United States and Europe, estimating the supply of serum albumin to be between 1.40 and 2.0 million kg from western countries prior to any purification or consideration of other plasma derived product manufacturing [9]. To further elaborate on this issue of demand, 130 plasma donations are required to manufacture enough intravenous immune globulin therapy for a single patient with primary immunodeficiency for one year [11].
Even if a reliable supply is identified, sourcing albumin from human serum for downstream cell propagation brings inherent risk of variability amongst lots/preparations and vendors that could translate to unpredictable downstream final product quality. It has been well noted that significant differences in the redox state and β-D-glucan (BDG) concentrations exist in 5% human serum albumin preparations from commercial suppliers suitable for clinical use [12]. Another group demonstrated through electrospray ionization-time of flight mass spectrometry that the Cys34-cysteinylated serum was highly variable amongst different commercial albumin preparations. As a result, the antioxidative and ligand binding functions of the albumins varied significantly in that preparations that were highly oxidized did not demonstrate the degree of functionality expected [13]. Indeed, we demonstrated significant differences in the RP-UPLC profiles of human serum albumins from different suppliers as evidenced by inconsistent percentages of oxidized and unmodified albumins. Others have suggested that different preparations of human serum albumin can have variable lengths. Albumins were found to have variable aspartyl-alanyl diketopiperazine (DA-DKP) content of human serum albumin and percentage of human serum albumin having lost its amino terminal dipeptide aspartyl alanyl (HSA-DA) were noted [14]. Combined with the variable oxidation states, DA-DKP was observed in significant amounts in human serum albumins tested [14]. DA-DKP was found to be partially responsible for the in vitro immunosuppressive effects observed in T Lymphocytes [14].
To further compound the variability issues, different suppliers use variable levels of stabilizers intended for pasteurization of native albumin. However, addition of stabilizers, such as octanoic acid, have been found to be problematic in select cell culture applications [15]. More interestingly, mitigation steps designed to remove these preservatives have been met with mixed success. It was demonstrated that the introduction of desalting steps intended to remove octanoic acid from albumin preparations performed well with specific vendors but failed to remove the compound from others as evidenced by toxic effects on murine blastocyst rates and positive identification of octanoic acid via mass spectrometry post desalting [15].
Raw material homogeneity aside, inclusion of blood sourced components into cell culture media carries an additional risk of potential contamination of the final product by adventitious agents [16,17]. Several safety measures have been implemented in the manufacture of human serum-derived components, such as prescreening voluntary donors, pooling large numbers of donors for contaminate virus dilution, and viral inactivation steps during purification and final packaging [18]. However, the risk of transmission of infectious agents cannot be eliminated as viral titer and virulence reduction steps can be escaped [18]. Hepatitis virus has been shown to be resistant to chemical and physical inactivation as serum contaminated with Hepatitis B virus was shown to be infectious in lab chimpanzees post pasteurization for 10 hours at 60⁰C [18]. However, high initial titers would be required to retain infectivity in the final product which lowers the probability of actual occurrence. To support this, there is no known case of virus transmission from purified serum albumin in man although cases of hepatitis B transmission have been identified in plasma protein fractions [18]. Nevertheless, the utilization of human plasma derived products represents a known risk of virus transmission due to the constant threat of new outbreaks and emergence of novel pathogens.
The importance of the risks associated with utilization of serum-derived albumin warrants the consideration of a recombinant alternative for inclusion in cell culture media to drive the innovative progress in cellular therapeutics. We have successfully expressed and purified a recombinant human albumin known as Cellastim S®. This highly pure, lipid rich albumin demonstrates excellent ability in expanding the growth of a variety of different cell types in vitro. Here, we provide proof of principal in the utility of Cellastim S® in mammalian cell culture by analyzing the expansion of human T Lymphocytes from peripheral blood of healthy donors. The data presented here shows that Cellastim S® exhibits equivalent ability to expand these cells compared to human serum albumin and modified lipid bovine albumin. Further, RP-UPLC and mass spectrometry demonstrate structural equivalence to human serum albumin and high consistency in the performance of individual lots. In conclusion, Cellastim S® is an attractive alternative to human serum-derived albumin and thus enables a higher degree of chemical definition and blood-free means of expanding therapeutic cell types.
Financial & competing interests disclosure
The authors are employees of InVitria the developer and manufacturer of Cellastim S®. No writing assistance was utilized in the production of this manuscript.
References
1. Lee P, Wu X. Review: Modifications of Human Serum Albumin and Their Binding Effect, Curr. Pharm. Des. 2015; 21(14): 1862–1865.
CrossRef
2. Yang F, Zhang Y, Liang H. Interactive Association of Drugs Binding to Human Serum Albumin, Int. J. Mol. Sci. 2014; 15(3): 3580–3595.
CrossRef
3. Taverna M, Marie A-L, Mira J-P, Guidet B. Specific antioxidant properties of human serum albumin, Ann. Intensive Care. 2004; 3: 4.
CrossRef
4. Sleep D, Cameron J, Evans RL. Albumin as a versatile platform for drug half-life extension, Biochim. Biophys. Acta BBA – Gen. Subj. 2013; 1830(12): 5526–5534.
CrossRef
5. Blázquez-Prunera, Díez MJ, Gajardo R, Grancha S. Human mesenchymal stem cells maintain their phenotype, multipotentiality, and genetic stability when cultured using a defined xeno-free human plasma fraction, Stem Cell Res. Ther., 2017; 8.
6. Zhang D, Nandi S, Bryan P. Expression, purification and characterization of recombinant human transferrin from rice (Oryza sativa L.), Protein Expr. Purif. 2010; 74(1): 69–79.
CrossRef
7. Randall Alfano. Albumin in cell culture media, presented at the Scale-up and Manufacturing of Cell-Based Therapies, San Diego, CA 2017.
8. Raoufinia R, Mota A, Keyhanvar N, Safari F, Shamekhi S, Abdolalizadeh J. Overview of albumin and its purification methods, Adv. Pharm. Bull. 2016; 6(4): 495–507.
CrossRef
9. Product Forecast for Serum Albumin, Marketing Research Bureau 2013.
10. Matejtschuk P, Dash CH, Gascoigne EW. Production of human albumin solution: a continually developing colloid, Br. J. Anaesth. 2000; 85(6): 887–895.
CrossRef
11. Baxter US.: Plasma Donors Save Lives. [Online]. Website
12. Nakae H, Tomida K, Kikuya Y, Okuyama M, Igarashi T. Comparison of quality of human serum albumin preparations in two pharmaceutical products, Acute Med. Surg. 2017; 4(3): 251–254.
CrossRef
13. Miyamura S, Imafuku T, Anraku M et al., Comparison of Posttranslational Modification and the Functional Impairment of Human Serum Albumin in Commercial Preparations, J. Pharm. Sci. 2016; 105(3): 1043–1049.
CrossRef
14. Bar-or D Thomas GW, Bar-Or R et al., Commercial human albumin preparations for clinical use are immunosuppressive in vitro, Crit. Care Med. 2006; 34(6): 1707–1712.
CrossRef
15. Leonard PH, Charlesworth MC, Benson L, Walker DL, Fredrickson JR, Morbeck DE, Variability in protein quality used for embryo culture: embryotoxicity of the stabilizer octanoic acid, Fertil. Steril. 2013; 100(2): 544–549.
CrossRef
16. Merten OW. Safety issues of animal products used in serum-free media, Dev. Biol. Stand. 1999; 99, 167–180.
17. The Case for Serum-Free Media, BioProcess International. 2003.
18. Erstad BL. Viral Infectivity of Albumin and Plasma Protein Fraction, Pharmacother. J. Hum. Pharmacol. Drug Ther. 1996; 16(6): 996–1001.
Affiliations
Atherly Pennybaker
InVitria, 320 E Vine Drive Suite 225, Fort Collins, CO 80524
apennybaker@invitria.com
Randall Alfano
InVitria, 320 E Vine Drive Suite 225, Fort Collins, CO 80524
ralfano@invitria.com
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</


