AAV capture purification scaling from bench to clinical manufacturing
Cell & Gene Therapy Insights 2024; 10(7), 965;
DOI: 10.18609/cgti.2024.110
Published: 27 August 2024
FastFacts
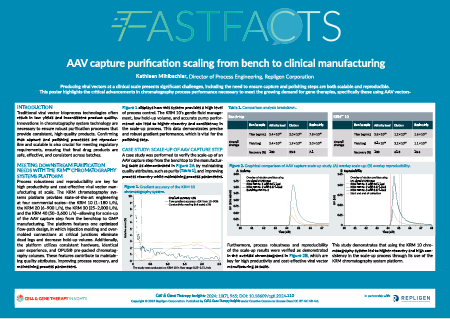 | Watch the video or view the poster to:
|
