It’s a match: cell line engineering for AAV manufacturing expands the options for therapeutic programs
Cell & Gene Therapy Insights 2024; 10(5), 879–888
DOI: 10.18609/cgti.2024.098
As AAV manufacturing enters its third generation, cell line engineering can enable enhanced productivity, scalability, and quality of viral vectors. A range of cell line options can allow therapy developers to tailor the choice for each therapeutic program and seamlessly transition from R&D through manufacturing as needs evolve. Furthermore, cell line engineering can tackle some of the inherent quality issues like host cell DNA (hcDNA) inside capsids, an impurity that cannot be removed in current downstream processing. This article will address how advanced engineered cell lines can empower researchers and developers to realize the full potential of gene therapy. New methods to harness cell line engineering to improve AAV through quality by design will be explored, alongside new performance data acquired with ELEVECTA™ cell lines.
How is the AAV manufacturing process evolving?
Over nearly 60 years of research into AAV, the manufacturing processes to produce the vector have greatly evolved. Early research into AAV was catalyzed by the discovery of HEK293 cells, which were originally adherent and grew in serum-rich media in 2D cultures. As the number of gene therapy clinical trials steadily increased in the early 2000s, leading to the first approvals in Europe in 2012 and the USA in 2017, the industry began implementing suspension culture for HEK293 cells to reduce costs and increase flexibility in line with the increasing demand. HEK293 cells are now adapted to serum-free suspension culture and can be grown in stirred tank bioreactors at much higher cell densities. However, both adherent and suspension-adapted HEK293 cells require triple transfection to produce AAV. This method relies on intensive manual intervention to add necessary genes via the transfection complex, is difficult to scale, and requires expensive reagents and plasmid DNA (pDNA) for every batch.
Now, AAV manufacturing has entered its third generation, in which a producer cell line is engineered by stably incorporating all necessary genes into the HEK293 host cell line genome. This enables HEK293 cells to grow in suspension freely and can be scaled to any volume, requiring only one induction step to start AAV production. Packaging cell lines require a transfection of a single plasmid for the gene of interest (GOI). Removing transient transfection as much as possible simplifies the process and reduces the need for expensive reagents and pDNA. This third-generation process still requires further optimization to replace the need for other modes of manufacture. Cytiva has launched a portfolio of cell lines to allow developers to choose how to best produce AAV to match their needs at a given time.
A cell line portfolio for differing needs
One cell line does not fit all needs. Triple transfection to produce AAV transiently is the foundational process for anyone starting therapy development, as it is quick, flexible, and requires little upfront investment. The ELEVECTA™ transient cell line is an off-the-shelf HEK293 cell line that has been adapted to suspension in-house by Cytiva. This provides the flexibility and speed needed at the early stages of therapy development. For those looking for a more strategic and future-proof method of producing AAV, stable cell lines, including packaging and producer cell lines, can meet those needs. A packaging cell line is an effective vehicle for screening multiple GOIs with a chosen capsid and can reduce plasmid costs and improve batch-to-batch consistency while simplifying the upstream process with single-plasmid transfection. The ELEVECTA producer cell line is a widely available stable producer cell line designed to remove raw material bottlenecks and batch-to-batch variability, and further simplify upstream processing by eliminating transfection.
When selecting the right cell line for AAV production, careful analysis of a therapy’s application to a given patient population must be made. Cytiva’s ELEVECTA transient cell line has been proven to work at 10 L scale and is suitable for use within rare indications with low dose requirements. The ELEVECTA packaging cell line is suited to reducing plasmid costs or for platform therapies that utilize the same serotype for several indications. Submitting one cell line to the regulators for multiple assets is expected to dramatically reduce time to clinic. The ELEVECTA producer cell line caters to therapies targeting large patient populations or requiring systemic doses. These therapies will be commercially viable only when a scalable manufacturing process becomes cost-effective, which can be enabled by stable producer cell lines. Transitioning developers from transient transfection into stable cell lines is the goal of the ELEVECTA cell line portfolio. Starting stable cell line development while in early clinical stages is recommended to ensure speed to clinic.
Engineering ELEVECTA transient cell lines for enhanced quality
The ELEVECTA transient cell line is the only known cell line that addresses minimizing the encapsidation of hcDNA. ELEVECTA cell lines are genetically modified to minimize hcDNA, an impurity that is resistant to DNase treatment and cannot be eliminated via downstream processing. ELEVECTA transient cell line shows ≤12.4 ng hcDNA per 1014 viral genomes (vg) in the 10 L scaled-up process, a 100-fold reduction over another commercially available cell line, as shown in Figure 1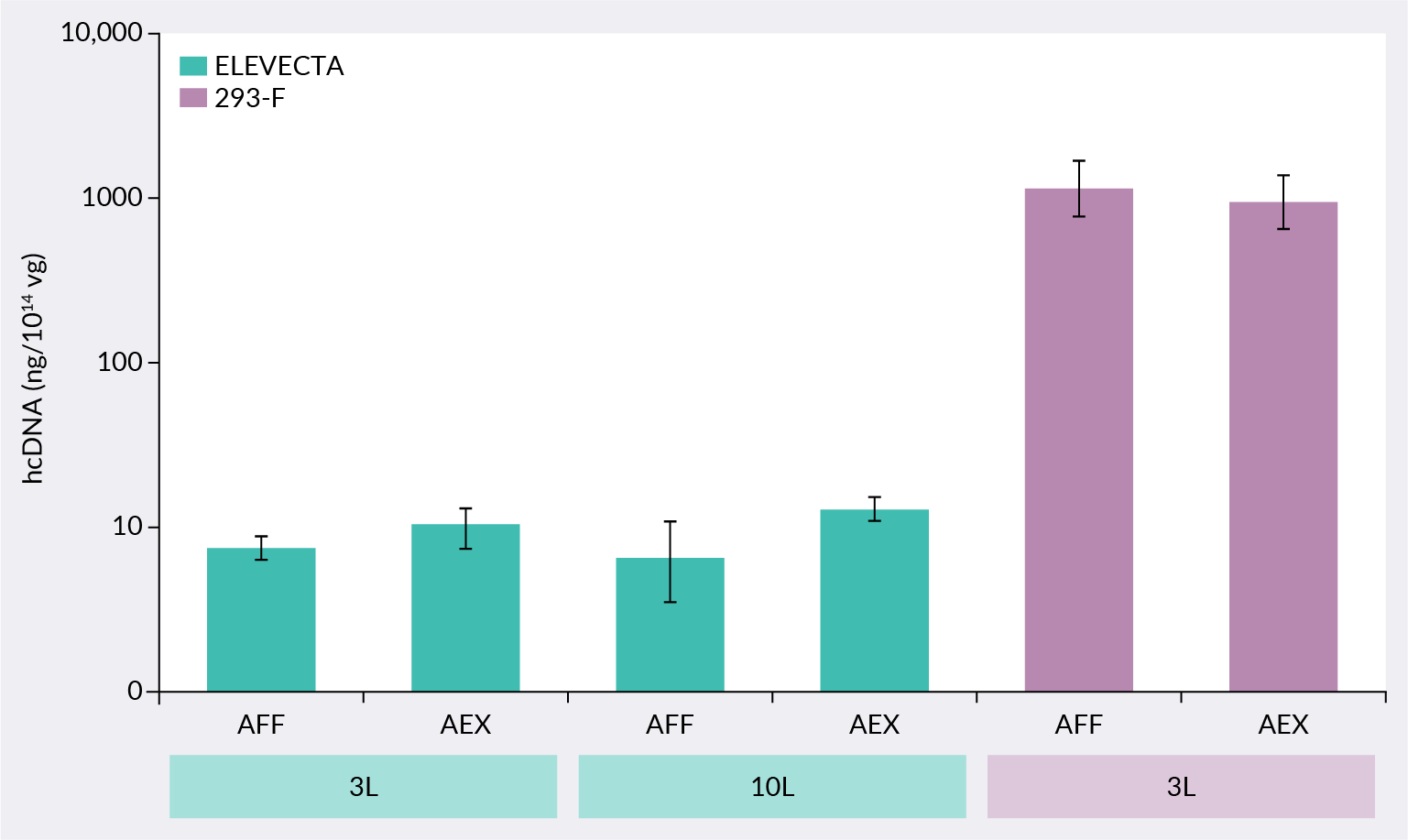 ELEVECTA transient cell line reduces encapsidated hcDNA.AFF: affinity samples, AEX: anion-exchange enriched samples..
ELEVECTA transient cell line reduces encapsidated hcDNA.AFF: affinity samples, AEX: anion-exchange enriched samples..
Regulatory guidance recommends reducing any non-vector DNA contamination in the final product. There is a regulatory expectation to control residual DNA to below 10 ng per administered dose. Residual DNA includes both DNA outside of viral capsids and encapsidated DNA impurities such as pDNA or hcDNA. Until now, however, the residual DNA guidelines have been difficult to meet for AAV therapies due to large quantities of encapsidated DNA impurities. This level of impurity often comes with recommended mitigations such as quality data, risk assessments, and control strategies. Reducing the total hcDNA to levels below the overall residual DNA guideline limits will substantially simplify these risk mitigation strategies. For more information on this feature, watch/read our Cell and Gene Therapy Insights Fast Facts on this topic.
Basic characteristics and scale-up data for the ELEVECTA transient cell line have been generated and are presented in Figure 2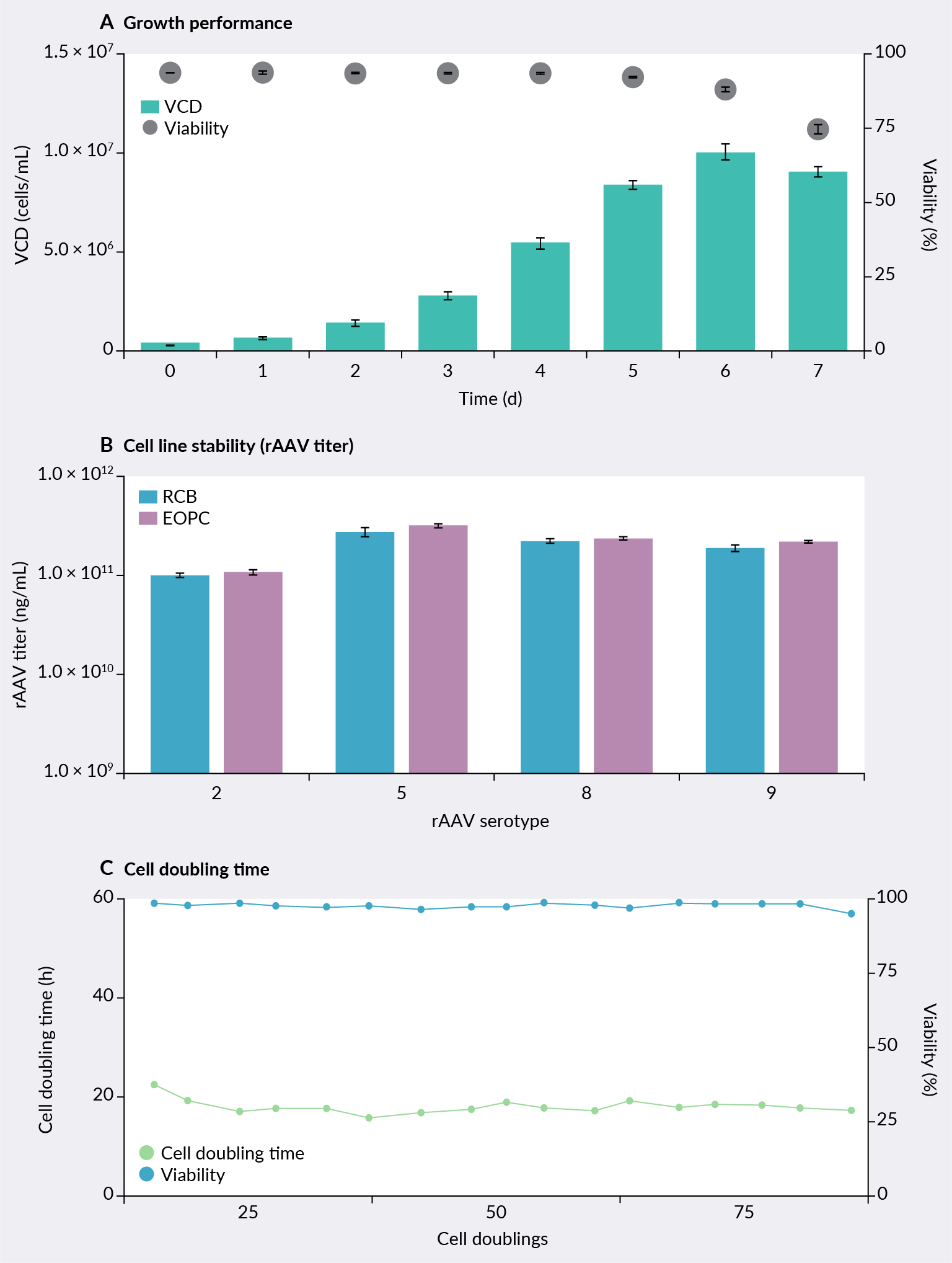 ELEVECTA transient cell line: basic characteristics.. This cell line is grown in HyClone™ prime expression medium specifically formulated to optimize cell growth and function. Growth performance was analyzed in batch mode, where peak viable cell densities of >1 × 107 cells/mL and an average cell doubling time of 20 hours were observed. To assess cell line stability, the ELEVECTA transient cell line underwent serial passage for 70 cell doublings and subsequently was tested for AAV productivity by transient transfection. Excellent stability in terms of subculture performance and AAV production performance for multiple serotypes was demonstrated.
ELEVECTA transient cell line: basic characteristics.. This cell line is grown in HyClone™ prime expression medium specifically formulated to optimize cell growth and function. Growth performance was analyzed in batch mode, where peak viable cell densities of >1 × 107 cells/mL and an average cell doubling time of 20 hours were observed. To assess cell line stability, the ELEVECTA transient cell line underwent serial passage for 70 cell doublings and subsequently was tested for AAV productivity by transient transfection. Excellent stability in terms of subculture performance and AAV production performance for multiple serotypes was demonstrated.
AAV production performance for multiple serotypes was also analyzed in a 15 mL microbioreactor, as shown in Figure 3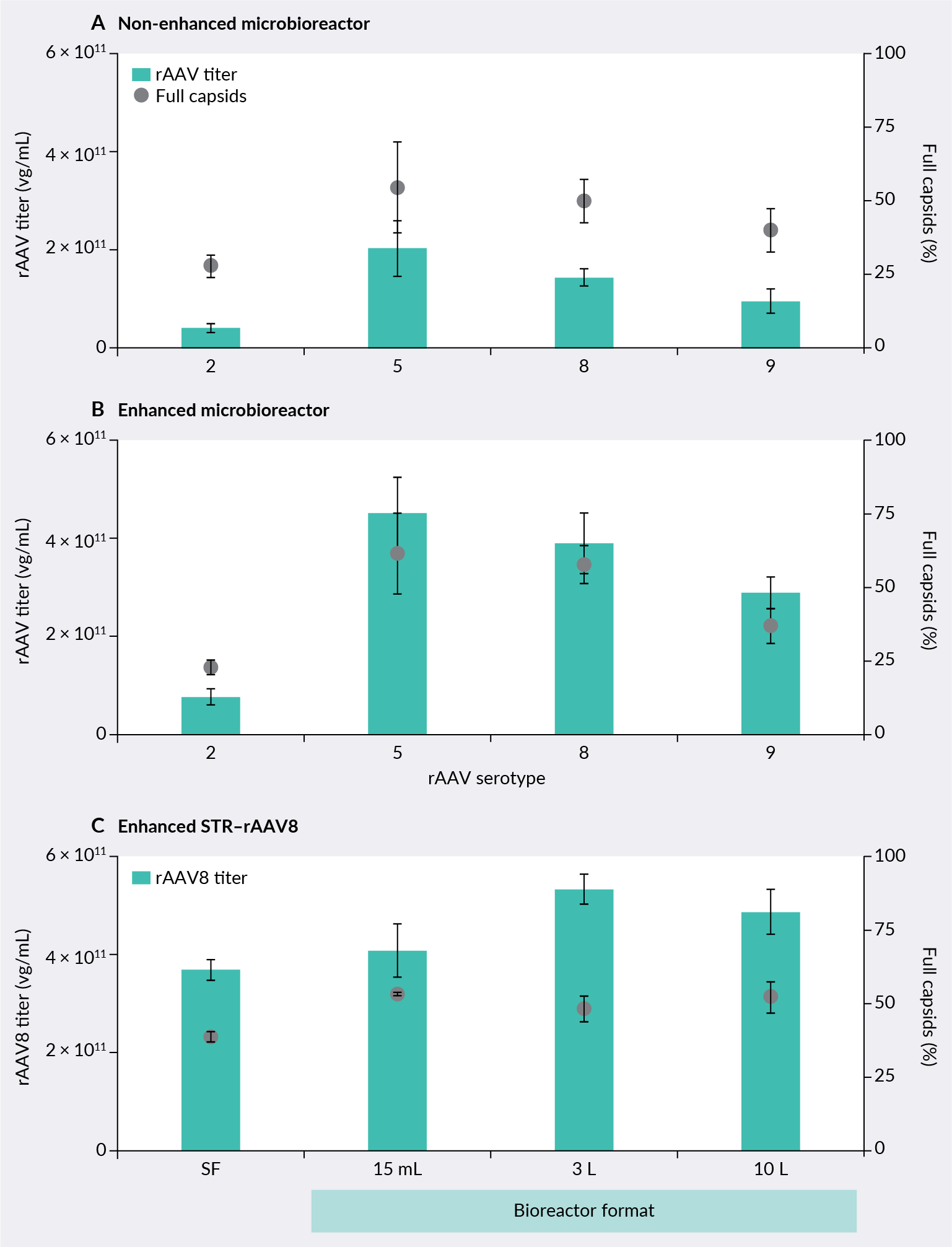 ELEVECTA transient cell line: scale-up.. Baseline process performance was compared against a commercially available AAV enhancer-supplemented process. AAV titers were analyzed by qPCR and ELISA and used to calculate packaging efficiencies. Cells were transfected using PEIMax® transfection reagent and a standard three plasmid system from Aldevron. High-titer AAV productivity and packaging efficiency were demonstrated for all serotypes. To evaluate process scalability, AAV8 production with an enhancer addition was analyzed in 3 L and 10 L stirred tank bioreactors. Robust and consistent performance in terms of growth, genomic titers, and packaging efficiency was observed throughout all production scales.
ELEVECTA transient cell line: scale-up.. Baseline process performance was compared against a commercially available AAV enhancer-supplemented process. AAV titers were analyzed by qPCR and ELISA and used to calculate packaging efficiencies. Cells were transfected using PEIMax® transfection reagent and a standard three plasmid system from Aldevron. High-titer AAV productivity and packaging efficiency were demonstrated for all serotypes. To evaluate process scalability, AAV8 production with an enhancer addition was analyzed in 3 L and 10 L stirred tank bioreactors. Robust and consistent performance in terms of growth, genomic titers, and packaging efficiency was observed throughout all production scales.
ELEVECTA stable cell lines: packaging and producer
The ELEVECTA stable cell lines are generated using Cytiva’s suspension-adapted parental HEK293 cell line. This is used to create an alpha cell line in which Rep and Helper genes are stably integrated under the Tet-On inducible promoter, allowing for controlled expression of Rep and Helper proteins, which are cytotoxic to the host cell. The alpha cell line undergoes a project-specific integration of cap genes to create a packaging cell line or one further stable integration of a GOI transgene to create a producer cell line.
ELEVECTA producer cell line performance is analyzed across the different cell line development stages. Figure 4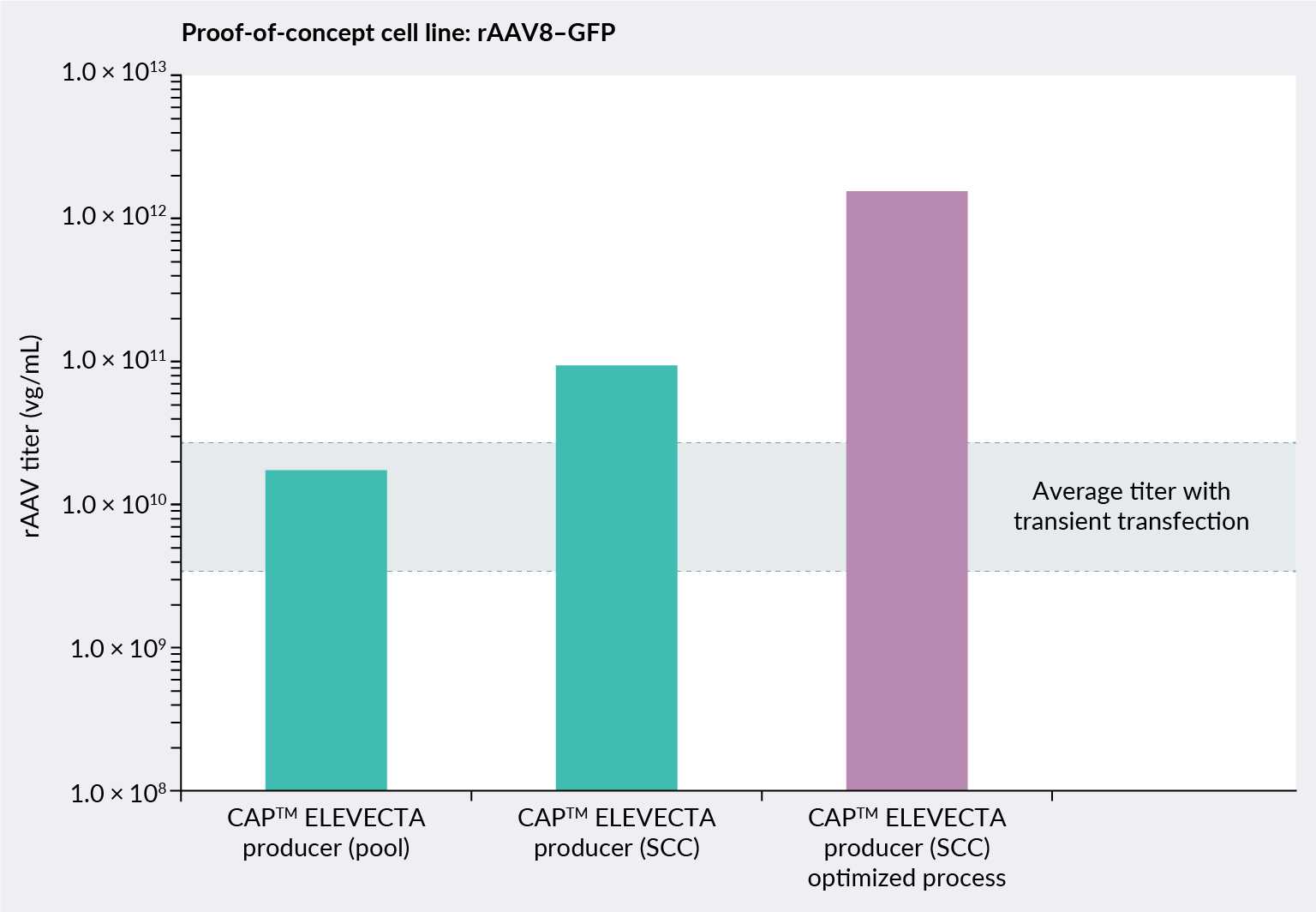 ELVECTA producer proof-of-concept cell line: rAAV8-GFP.SCC: single cell clone. shows how the performance of the rAAV8 proof-of-concept cell line improves when comparing a producer pool with a single cell clone (SCC). Based on a standardized manufacturing system, the upstream process can be further intensified to reach higher yields.
ELVECTA producer proof-of-concept cell line: rAAV8-GFP.SCC: single cell clone. shows how the performance of the rAAV8 proof-of-concept cell line improves when comparing a producer pool with a single cell clone (SCC). Based on a standardized manufacturing system, the upstream process can be further intensified to reach higher yields.
The AAV2 proof-of-concept cell line shows similar performance trends when comparing pool and SCC. Figure 5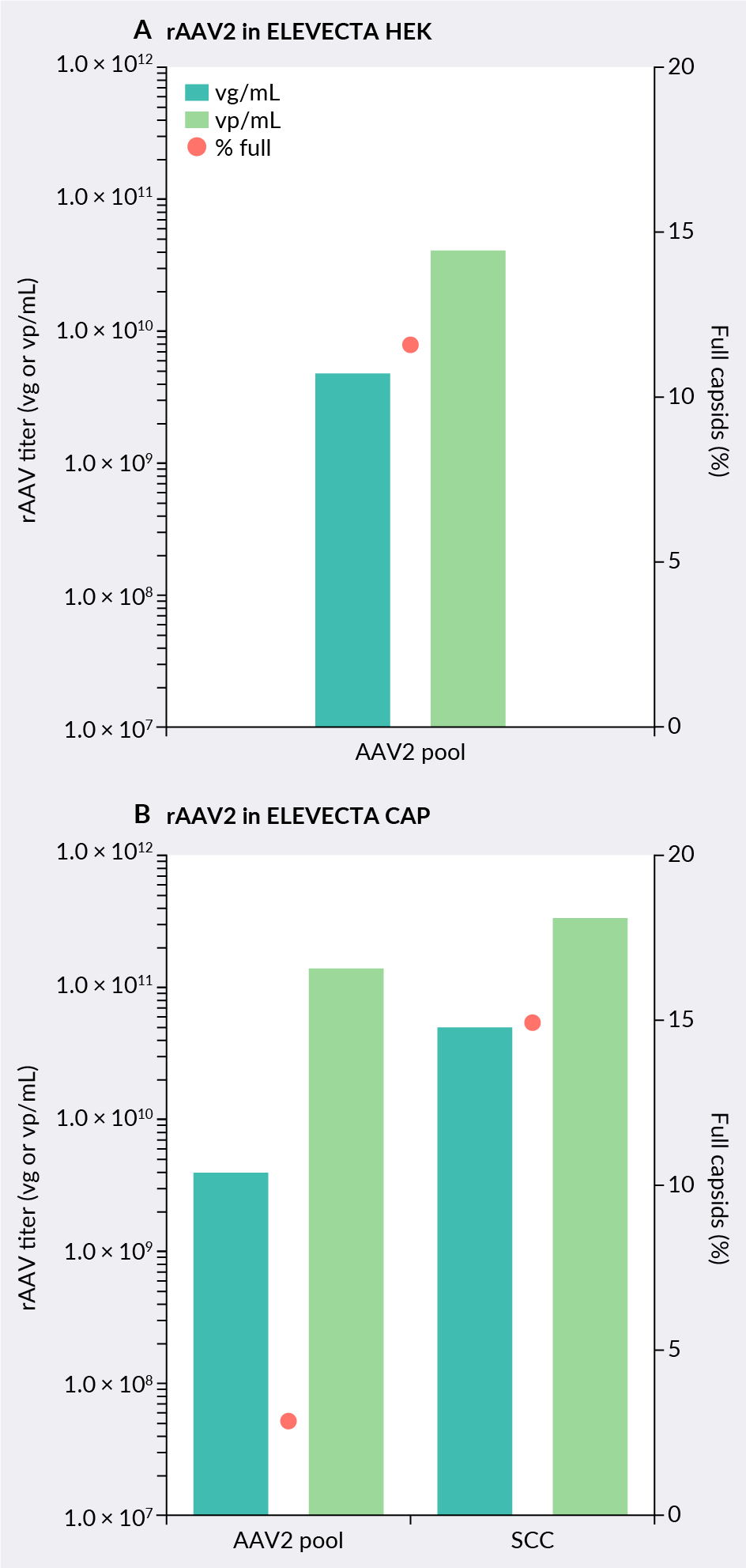 ELEVECTA rAAV2 producer proof-of-concept cell line on HEK and CAP (GFP-Luc). shows two proof-of-concept cell lines: one generated on the HEK293 parental cell line and the other on the CAP parental cell line. This in-house data was obtained before any process optimization, showing viral yields of a difficult-to-produce AAV2 at industry average levels.
ELEVECTA rAAV2 producer proof-of-concept cell line on HEK and CAP (GFP-Luc). shows two proof-of-concept cell lines: one generated on the HEK293 parental cell line and the other on the CAP parental cell line. This in-house data was obtained before any process optimization, showing viral yields of a difficult-to-produce AAV2 at industry average levels.
Summary
Generating a suitable cell line is not done in isolation; a full suite of products and services can be leveraged by clients to increase cell line performance. Off-the-shelf and custom HyClone™ media development can ensure optimal cell line performance. Fast Trak™ process development services can help select the best-performing clones and process conditions to be used at large-scale manufacturing to maximize success when cell lines are transferred to a manufacturing facility. Additionally, stirred-tank bioreactors and ÄKTA chromatography systems are integral tools to generate valuable data on cell lines.
The ability to choose the right cell line for a given therapy journey stage is critical for success. Cytiva’s cell lines are engineered to simplify the manufacturing process and to address some of the most pressing quality challenges, such as encapsidated hcDNA. These stable cell lines continue to be refined to further improve their performance and incorporate features to address other challenges associated with AAV manufacturing.
Q&A
Dovilé Woods
Have you checked the infectivity of the ELEVECTA transient cell line, and what assays were used?
DW: Our AAV bioreactor lysates were subjected to affinity and ion exchange chromatography enrichment for full capsids, and then further analyzed for potency. Our ELEVECTA transient cell line-derived AAV8 material was comparable in potency levels across 3 and 10 L scales to another commercially available cell line, which was 293F-derived AAV8 material. We are confident that our cell line can generate infectious particles. Infectious titer was determined using cell-based assay which measures the expression of the GOI, in our case GFP as a model molecule.
How do you support clients transitioning from transient to producer cell lines in the middle of a clinical trial?
DW: This is something that we are working quite intensively on. At Cytiva, we believe that the future of AAV manufacturing is stable cell lines, so we recognize that there will be a shift to those cell lines in the industry. How you approach switching to the cell lines is important. We have a strong regulatory and quality team who have supported many clients moving through clinical trials and making changes to their processes. The regulatory landscape is familiar to us; our teams have supported clients in preparing for meetings with the US FDA, for example.
We hold a lot of experimental developmental quality data on our cell lines, as well as a fully documented history for every cell line we offer to clients. Our parental CAP cell line for transient production has a drug master file (DMF) submitted to the FDA. Our HEK293-based ELEVECTA transient cell line will also have a DMF filed with the FDA imminently, allowing clients to seamlessly cross-reference all those documents.
We are also working on an in-depth internal comparability study with our cell lines to best prepare our internal data and fill any gaps we may have so that when a client is undergoing this change, we are ready to hand over as much information as possible in the most organized manner.
How do you select clones where titer or fullness needs to be prioritized?
DW: For us, this is a common question and with every client project, we arrive at this decision point. This is an individual choice that can be up to our clients. However, for our internal R&D activities and for promoting the cell lines’ performance, we prioritize fullness wherever possible. This is the key quality attribute for our cell lines. This needs to be balanced with titer, though, as you cannot have one without the other.
How do regulators view new stable AAV lines versus established transient methods with years of IND data?
DW: This is becoming a less important consideration amongst our clients in our conversations about stable cell lines. New technologies are coming out every day and we believe that the FDA will not preferentially view old versus new. What matters is how well understood, characterized, and documented that new technology is.
We are seeing a shift in mindset in the industry where stable producer cell lines are not a novelty, especially as they have been used widely with monoclonal antibodies (mAbs). The degree to which we need to define them is, however, is higher with AAV than with mAbs.
Biography
Dovilé Woods has held various engineering and management positions in the biotechnology sector. She has spent most of her career developing large-scale manufacturing enterprise solutions for biotechnology customers globally, for mAbs and gene therapy production. Dovilé has chemical engineering degrees from Newcastle University, Newcastle, UK and Delft University of Technology, Delft, Netherlands.
Affiliation
Dovilé Woods
Global Product Manager,
Cell Line Development (Viral Vectors),
Cytiva,
Amersham, UK
Authorship & Conflict of Interest
Disclaimer: ELEVECTA transient cell line and HyClone prime expression media will be available soon.
Contributions: The named author takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author has no conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2024 Cytiva. Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited. This article was based on a webinar, which can be found here.
Webinar conducted: May 28, 2024; Revised manuscript received: Jul 11, 2024;
Publication date: Aug 27, 2024.


