Strategies to Control CAR-T Cell Therapy: Perspective on Next-Generation CARs
Cell Gene Therapy Insights 2018; 4(4), 275-285.
10.18609/cgti.2018.028
Chimeric antigen receptor T (CAR-T) cells have produced remarkable results in clinical trials, resulting in the recent FDA approval of the first two products, Kymriah and Yescarta, for the treatment of B-cell malignancies. However, clinical experiences of severe adverse events, relapses related to antigen loss and the dearth of successes in solid tumors have defined the challenges to advancing the field. Recently, an explosive growth in synthetic biology strategies to program control into CAR-T cells has created an armamentarium of methods to overcome these challenges. Here we provide an overview of the types of control systems in these next generation CAR-T platforms and provide a perspective on how they address the delicate balance of efficacy and safety with engineered T cells.
Submitted for peer review: Mar 19 2018 Published: May 14 2018
Introduction
Synthetic biology approaches to engineered cellular immunotherapies are transforming the way physicians treat cancer [1–4]. Among these therapies, chimeric antigen receptor T-cell therapy (CAR-T) has generated the greatest enthusiasm, resulting in the approval of the first gene therapy products in the USA and ushering in a new era of medicine [5]. CAR-T cells are engineered by transduction of autologous or allogeneic T cells with a gene that encodes a fusion protein comprising an extracellular antigen binding domain (a single chain variable fragment [scFv] of a monoclonal antibody) linked to intracellular domains that trigger T-cell effector functions (Figure 1) [6]. This engineering reprograms the cells to recognize a defined antigen expressing target cell in an HLA-independent manner and has clear advantages over traditional therapeutics in the exploitation of the T-cell’s natural ability to autonomously carry out seek and destroy missions, move freely from one tissue to another, and proliferate rapidly in response to a challenge [7]. CAR-T cells can also eliminate chemotherapeutic resistant cells, cancer stem cells and cells that have escaped recognition by endogenous immune surveillance [8,9]. The result is induction of durable, minimal residual disease (MRD) negative responses in patients who have failed multiple lines of prior therapy [8].
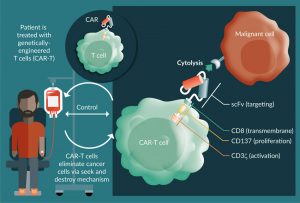
Figure 1: Chimeric Antigen Receptor T cell therapy approach. Left side: Genetically engineered T-cells are infused into patient to eliminate cancer cells. Right side: Schematic of a chimeric antigen receptor based on scFv (antigen binding domain)- transmembrane (CD8)-stimulatory (CD3) and costimulatory domains (CD137). Figure adapted from Essand M and Loskog AS [6].
The central tenet of CAR-T cell engineering is to recapitulate the complex native T-cell functions to recognize, activate, lyse target and expand self, and is a triumph of minimalistic design. However, through this lens, CARs can also be viewed as a ‘dominant bypass mutation’ in that the CAR circumvents natural homeostatic mechanisms of immune regulation [1]. This unidimensional nature of the ‘living drug while at once powerful, is beset by challenges related to the lack of control. Here we investigate what these challenges are and how engineering molecular mechanisms of control affords the opportunity to rebalance the equation in ways that benefit both safety and efficacy.
CAR-T cell challenges
Specificity in target cell recognition is ostensibly the first nexus of potency and toxicity. Lack of fidelity for the tumor cell can cause two types of toxicity: off-target, off-tumor and on-target, off-tumor. Off-target, off-tumor toxicity results from binding of the scFv domain to the incorrect antigen. Although this type of toxicity is a significant concern for affinity matured T-cell receptor (TCR) engineered cells [10], it is comparatively less common for CARs as majority of scFv’s are derived from validated antibody clones and established methods are in place testing antibody cross reactivity.
On-target, off-tumor toxicity is a larger liability for CARs. In the case of CD19-targeted CARs such as Kymriah and Yescarta, CD19 is shared on healthy and neoplastic B cells. Loss of healthy B cells is considered tolerable collateral damage, even when long-term B-cell aplasia is induced in some patients [11,12]. However, it is appreciated that similar targets for solid tumors may not exist. These antigens are frequently shared with vital tissues and correspondingly, on-target, off-tumor reactivity can be fatal. For example, CAR-T cells targeting Her2 (ErbB2) recognized low levels of Her2 in cardiopulmonary tissue and resulted in a patient fatality [13]. Notably this fatal toxicity is not commonly found with trastuzumab, the standard of care for Her2-positive breast cancer [14]. This highlights the new safety concerns inherent to the potency of CAR-T cells. Control mechanisms to tune activity within a therapeutic index or that use logic gates to discriminate tumor tissue from normal tissue may allow the targeting of antigens for solid tumors that are not possible with non-controlled approaches.
CAR-T cell therapy clinical outcomes are further challenged by loss of target antigen on malignant cells. In trials with CTL019, up to 30% of patients who relapsed were found to have CD19-negative B cell leukemia [15]. A method that controls target antigen specificity could allow for redirection of the CAR-T cells in the patient to CD22, for example, to eliminate CD19 negative relapsing disease [16].
The next major challenge in CAR-T cell engineering is T-cell activation, expansion and persistence. Activation and expansion must afford a sufficient effector to target cell ratio necessary to eliminate tumor cells but without causing a run-away response that can injure the patient. Two of the most serious safety risks associated with this are cytokine release syndrome (CRS) and CAR‑T-cell-related encephalopathy syndrome (CRES) [17–19]. The anti-IL-6 mAb tocilizumab is effective at mitigating CRS; however, CRS and CRES have contributed to multiple patient deaths and thus remains a concern [20]. Control over activation and expansion post-adoptive transfer of the cells back to the patient can be a critical factor in avoiding these toxicities.
Persistence can be a double edged-sword for CAR-T cells. On one side, persistence may afford durable, MRD-negative responses, on the other, it contributes to permanent B-cell aplasia when targeting CD19 and could lead to chronic toxicities in targeting solid tumor antigens [18]. However, it’s unknown how long CAR-T cells must persist to achieve complete target cell elimination and it’s likely different for each indication. Here, mechanisms that can turn CAR-Ts on and off ‘at will’ may prevent long-term concerns like B-cell aplasia and provide the appropriate duration of activity for each indication.
The final challenge that confronts CAR-T cell therapy is the intratumoral trafficking and immunosuppression by a hostile tumor microenvironment (TME) [21]. High interstitial tumor pressure, physical fibrotic barriers, hypoxia, metabolic restrictions as well as immunosuppressive ligands and cytokines are just a few of the barriers facing CAR-T cells. This dark matter of cancer immunity is at the forefront of translating the successes observed in hematological malignancies to solid tumors. Which one of these factors must be overcome first in order to tip the scales in favor of tumor immunity has yet to be deciphered; however, control mechanisms can provide an advantage. For example, control of cytokine release or induction of pro-survival factors may be selectively turned on in the presence of the TME or by the co-administration of a drug. This type of an armored approach is expected to allow the CAR-T cell to better penetrate high fibrotic and immunosuppressive environments.
How to control challenges
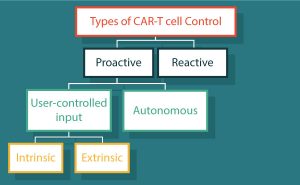
The bulk of challenges confronting CAR-T cells stems from the entropic costs of a living drug. Design of control mechanisms offer an opportunity to return decision making back to the system that was circumvented in the ‘dominant bypass mutation’. Types of control can be divided into two main categories (Table 1 & Figure 2): reactive control refers to an action or method applied in response to an undesirable effect; and proactive control meaning the intention of preventing the undesirable effect before it begins. Here we address how each confronts these multi-dimensional challenges.
| Table 1: Types of control of CAR-T cells | ||
|---|---|---|
| Reactive | ||
| Aimed to treat symptoms | Tocilizumab and corticosteroids [20–22] | |
| Kill switch | huEGFRt [24], RQR8 [25] | |
| Suicide genes | HSV-TK [28–30], CaspaCIDe [31–33] | |
| Proactive | ||
| Autonomous | NOT-gate | iCAR [35] |
| OR-Gate | TanCARs [36] | |
| AND-Gate | Dual CAR [37] | |
| Logic systems | SynNotch [38] | |
| User-defined | Intrinsic | FKBP-based CID [40–42] |
| Extrinsic | Switchable CARs [43–48] | |
Reactive control/mitigation
The first form of control for engineered cells is reactive control – an attempt to mitigate damage in response to an adverse or unexpected result. Currently, managing CRS with tocilizumab and corticosteroid administration is a form of reactive control used to treat symptoms related to T-cell overactivation and macrophage activation syndrome [20]. However, significant evidence argues for the prophylactic treatment of patients with tocilizumab, which has been shown to not affect the efficacy of CAR-T cells [22]. The desire to move this therapy to a proactive form of control underscores the importance of addressing this safety issue [23].
The most prominent form of engineered reactive control is a ‘kill switch’. In this case, cells are engineered with a trigger to eliminate them in the case of a specific adverse event (SAE) or at the first harbinger of danger to the patient. For example, several groups have designed epitope markers expressed from the CAR vector in a bicistronic format. Marked cells can then be eliminated by an approved monoclonal antibody. This has been designed with a truncated EGFR variant (huEGFRt), that renders it inert, but preserves the conformationally intact binding epitope for cetuximab (Erbitux) allowing clearance of CAR-T cells [24]. Similarly, the CD20 epitope target of rituximab has been buried into a compact marker that combines it with a CD34 epitope for efficient cell sorting, called RQR8 [25]. This strategy has been demonstrated to reverse CART-19-mediated B-cell aplasia in preclinical mouse models and may be an option for patients to eliminate engineered cells after a period of remission [26].
A second approach to the ‘kill switch’ technique has employed a suicide gene system [27]. While incorporation of the herpes simplex virus-thymidine kinase (HSV-TK) for elimination of cells with ganciclovir has historically been the choice construct for this purpose in clinical cell therapy investigations [28,29], HSV-TK has several limitations including the relatively slow rate of cell elimination (3 days) that has driven the development of other solutions [30]. The most prominent suicide gene currently in T cell-based immunotherapies is a chemically inducible dimerization (CID) of an engineered caspase 9 (iCas9, CaspaCIDe). This fusion protein comprises the proteolytic domain of caspase 9 fused in-frame to the FK506 binding protein. Treatment with small molecule analogs of rapamycin (rapalogs such as AP1903) causes dimerization of caspase 9 and subsequent cell apoptosis [31–33]. This strategy has demonstrated extraordinary efficiency in clinical trials of patients receiving haploidentical hematopoietic stem cell transplantation, demonstrating the rapid elimination of graft versus host disease [34].
These methods of reactive control can be very effective at limiting adverse events. However, just like a car needs more than gas and a brake pedal, so too will CARs need more control levers, to navigate the full breadth of the challenges.
Proactive, autonomous control
One strategy to proactively control CAR-T cells is to engineer autonomous decision-making capabilities into the cell to complement the CAR. Genetic circuits can create logic gates that allow the cells to perform Boolean operations in response to stimuli. An early example of this was a NOT-gate created by chimerizing an extracellular scFv with the intracellular domain of immuno-inhibitory receptor CTLA-4 or PD-1 [35], referred to as an iCAR. When expressed in the same cell as a conventional CD3z-based CAR, it allows input from two antigens to decide in an antigen A NOT B operation. This is expected to be important in solid tumors where few truly tumor-specific antigens exist, and nearly all are shared with healthy tissues. It has been hypothesized that tumor suppressor genes, expressed on normal tissue, could be used as antigen B; however, this has yet to be reduced to practice.
Engineering of OR-gates is relatively straightforward for CAR-T cells. For example, two conventional CARs can be transduced into the same cell to create an antigen A OR B operation. A more sophisticated approach to this is the TanCARs where two scFvs are encoded in tandem in the same CAR [36]. This is expected to be useful in targeting heterogeneous neoplastic diseases or antigen-loss relapse events. For example, in CD19 antigen loss relapse for B-cell malignancies, a TanCAR that targets both CD19 and CD20 or CD22 could prevent tumor escape.
Split CARs create AND-gates by splitting the activation and costimulatory domains, each having their own extracellular scFv, and by association, ability to recognize distinct targets [37]. In this way, each scFv must bind to the target cell and create a productive synapse to achieve full activation. This is expected to allow more precise discrimination of tumoral from healthy tissue.
A recent example of a highly versatile logic system is the SynNotch platform [38]. The components of this system diverge from the above examples in that they use an orthogonal signal cascade derived from modular notch receptors to create a range of customized response behaviors to contextual cues. This can be applied to expression of nearly any transgene including cytokines or local production of a therapeutic antibody. Inputs can be used combinatorially to create genetic T-cell circuits that can not only enhance recognition but provide extra stimulus to overcome the suppressive TME or promote expansion when needed. While it is still early for this technology, the ability to produce highly precise ‘armored’ CARs may create opportunities for solid tumor penetrating CAR-T cells.
Proactive, user-defined control
While autonomous control seeks to give decision-making capacity back to the T cell, user-defined control seeks to give physicians remote control over the engineered cells. In this way, the activation of the cell is controlled by the pharmacokinetics of a small molecule or biologic. This has the advantage of restoring pharmacological control over an exponentially expanding cell.
Proactive, user-defined control can be further divided into intrinsic mechanisms that alter the intracellular signaling cascades within the cell and extrinsic mechanisms that modulate the interaction of the CAR with other cells. Intrinsic mechanisms are dominated by the FKBP-based CID approach [39]. In these examples, the CAR is split at various sites, with each half fused in-frame to an FKBP domain. The mechanism is reminiscent of the iCas9 system, but with a stimulatory effect, rendering the CAR natively inactive until in the presence of a rapalog. Specific reductions to practice include GoCAR-T (Bellicum) [40], THROTTLE (CDL/Gilead) [41], or DARIC (Bluebird) [42]. Each differs in the orientation and location of the CAR and FKBP domain (i.e., intracellular or extracellular) but with roots in the same CID foundation. Conceptually this method of titrating the CAR “on” is expected to be safer than turning the CAR “off” and may facilitate the entry into more broadly expressed targets.
Extrinsic controls do not alter the core CAR machinery, but rather act as an intermediary ‘switch’ to govern the interaction of the CAR and the target cell. A potential advantage of this methodology is that it leverages the existing understanding and clinical experience of the conventional CAR-T cell design with regards to cell manufacturing and expected cell behavior.
To provide extrinsic control, the extracellular scFv of the CAR is designed to target the switch molecule instead of directly targeting the tumor antigen. The switch then, in turn, targets the tumor antigen and thus serves as a bridge between the CAR and the target cell. In this way, the cells (inactive in the absence of the switch) are provided first, and the switch delivered subsequently to tune or titrate the CAR-T cell activity. One way this has been designed is by replacing the scFv with a high affinity variant of the Fc-receptor (CD16) [43]. Redirection to the tumor target is accomplished using off-the shelf monoclonal antibodies; however, as the Fc-receptor can bind to any antibody, it is conceivable that off-target reactivity from endogenous Ig levels could result in unexpected side effects.
To address this, fully orthogonal systems have also been designed in such a way that the CAR and the switch do not cross react with other antibodies or immune components. This was initially accomplished using scFv’s that targeted a small molecule such as FITC or Biotin, and a monoclonal antibody conjugated to the small molecule that served as the switch [44–46]. More recently, however, fully recombinant antibody-based switches have been developed using a CAR that targets a peptide epitope that is buried in the monoclonal antibody [47,48].
These extrinsic mechanisms of control offer a host of advantages, none more important than the concept that the CAR is natively off, and is therefore wholly dependent on the switch for activity. In this way, the full potential of the dynamic range of activity can be exploited which may not be fully realized with intrinsic systems that may have a basal level of activation in the absence of the activation agent [49]. Because the CAR is agnostic to antigen it can also be viewed as a universal system. In this way it can leverage a hardware and software approach, in that the cells (hardware) can be reprogramed by addition of a switch (software) against any antigen target. This is expected to be critically important in combating heterogenous neoplastic diseases and antigen loss relapse mutations, which in this case could be treated by providing the appropriate switch molecule rather than re-engineering a different CAR.
Translational insight
To date, the lion’s share of T-cell engineering has focused on potentiating efficacy with comparably less focus on regulation of activity and safety. More recently it has been appreciated that it is incumbent on synthetic immunologists to engineer molecular switches in order to control cell behavior. However, the success of conventional CARs has set a high bar for success of these ‘next-generation’ technologies. The first major challenge is that there are few mouse models predictive of the clinical experience [50]. Most preclinical models fail to recapitulate toxicity; thus, demonstration of safety requires surrogate readouts, and ultimately needs to be empirically demonstrated in humans [13,50]. The same is true for methods of enhancing efficacy in the context of solid tumors as xenograft models in immunodeficient mice – the workhorse model for this field – fail to capture this key suppressive factor. Surrogate models using engineered murine cells in syngeneic murine hosts may provide one answer to this challenge, but nevertheless are limited by differences in human and murine immunology and fail to fully recapitulate toxicities [51]. Few models of CAR-T cells in non-human primates have been described; however, this may be key to advancing the field [52].
The lack of preclinical models has also elicited key questions. For example, it remains to be understood whether the use of mAbs as kill switches can be deployed with enough speed to head-off a severe adverse event once started. Antibody-based kill switches have not been tested to avert an SAE crisis in the clinic and notably have been included in products that have still resulted in fatal reactivity. In the case of control switches that turn ‘on’ CAR-Ts, a major question to be answered is what happens to the CAR-T cells when they are off? Does the lack of signaling result in elimination of engineered cells or do the cells enter into a persistent resident memory population, waiting to be recalled at a later time? The kinetics of small molecule and antibody-based switches also raises questions – as CAR-T cells expand, so do the pharmacological targets – does this change the exposure of these molecules, and how should dosing be tied to CAR-T cell levels?
Finally, cost of goods is a significant challenge that CAR field must overcome to expand outside of specialized centers of care and broaden patient access. This challenge is a practical one that will need to be addressed by hospitals, payers and logistics, but that may also be programed at the level of the cell. For example, off-the shelf CAR-T cells may be enabled by allogeneic engineering strategies. This would significantly decrease cost and expand patient access but is accompanied by its own host of challenges [53]. On the other hand, certain types of control, such as those which define CAR antigen specificity, can also be viewed as ‘universal’ in that they are antigen agnostic and can be used across a wide range of indications. In this way, a standardized CAR-T cell could obviate the need to reconstruct a new CAR for each antigen target and would substantially lower the cost and time of ‘bench-to-bedside’ development.
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Roybal KT, Lim WA. Synthetic Immunology: Hacking Immune Cells to Expand Their Therapeutic Capabilities. Annu. Rev. Immunol. 2017; 35: 229–53.
CrossRef
2. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359(6382): 1361–5.
CrossRef
3. June CH, Maus MV, Plesa G et al. Engineered T cells for cancer therapy. Cancer Immunol. Immunother. 2014; 63(9): 969–75.
CrossRef
4. Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013; 39(1): 49–60.
CrossRef
5. First-Ever CAR T-cell Therapy Approved in U.S. Cancer Discov. 2017; 7(10): OF1.
6. Essand M, Loskog AS. Genetically engineered T cells for the treatment of cancer. J. Intern. Med. 2013; 273(2): 166–81.
CrossRef
7. Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016; 16(9): 566–81.
CrossRef
8. Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 2016; 14(10): 802–8.
9. Guo Y, Feng K, Wang Y, Han W. Targeting cancer stem cells by using chimeric antigen receptor-modified T cells: a potential and curable approach for cancer treatment. Protein Cell. 2017.
CrossRef
10. Linette GP, Stadtmauer EA, Maus MV et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013; 122(6): 863–71.
CrossRef
11. Kochenderfer JN, Dudley ME, Feldman SA et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119(12): 2709–20.
CrossRef
12. Kochenderfer JN, Wilson WH, Janik JE et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116(20): 4099–102.
CrossRef
13. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010; 18(4): 843–51.
CrossRef
14. Cameron D, Piccart-Gebhart MJ, Gelber RD et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017; 389(10075): 1195–205.
CrossRef
15. Maude SL, Frey N, Shaw PA et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014; 371(16): 1507–17.
CrossRef
16. Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016; 4(6): 498–508.
CrossRef
17. Hay KA, Hanafi LA, Li D et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017; 130(21): 2295–306.
CrossRef
18. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 2016; 3: 16011.
CrossRef
19. Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 2010; 18(4): 666–8.
CrossRef
20. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014; 20(2): 119–22.
CrossRef
21. Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol. Ther. Oncolytics 2016; 3: 16006.
CrossRef
22. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016; 127(26): 3321–30.
CrossRef
23. Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015; 125(26): 4017–23.
CrossRef
24. Wang X, Chang WC, Wong CW et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011; 118(5): 1255–63.
CrossRef
25. Philip B, Kokalaki E, Mekkaoui L et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 2014; 124(8): 1277–87.
CrossRef
26. Paszkiewicz PJ, Frassle SP, Srivastava S et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Invest. 2016; 126(11): 4262–72.
CrossRef
27. Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front. Pharmacol. 2014; 5: 254.
CrossRef
28. Greco R, Oliveira G, Stanghellini MT et al. Improving the safety of cell therapy with the TK-suicide gene. Front. Pharmacol. 2015; 6: 95.
CrossRef
29. Bonini C, Ferrari G, Verzeletti S et al. HSV-TK Gene Transfer into Donor Lymphocytes for Control of Allogeneic Graft-Versus-Leukemia. Science 1997; 276(5319): 1719–24.
CrossRef
30. Marin V, Cribioli E, Philip B et al. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum. Gene Ther. Methods. 2012; 23(6): 376–86.
CrossRef
31. Straathof KC, Pule MA, Yotnda P et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 2005; 105(11): 4247–54.
CrossRef
32. Clackson T, Yang W, Rozamus LW et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl Acad. Sci. USA 1998; 95(18): 10437–42.
CrossRef
33. Di Stasi A, Tey SK, Dotti G et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011; 365(18): 1673–83.
CrossRef
34. Zhou X, Dotti G, Krance RA et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood 2015; 125(26): 4103–13.
CrossRef
35. Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013; 5(215): 215ra172.
CrossRef
36. Grada Z, Hegde M, Byrd T et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol. Ther. Nucleic Acids 2013; 2: e105.
CrossRef
37. Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013; 31(1): 71–5.
CrossRef
38. Roybal KT, Rupp LJ, Morsut L et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016; 164(4): 770–9.
CrossRef
39. Liberles SD, Diver ST, Austin DJ, Schreiber SL. Inducible gene expression and protein translocation using nontoxic ligands identified by a mammalian three-hybrid screen. Proc. Natl Acad. Sci. USA 1997; 94(15): 7825–30.
CrossRef
40. Mata M, Gerken C, Nguyen P, Krenciute G, Spencer DM, Gottschalk S. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov. 2017; 7(11): 1306–19.
CrossRef
41. Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 2015; 350(6258): aab4077.
CrossRef
42. Leung W-H, Certo M, Horton H et al. Small Molecule-Regulated Antigen Recognition System for Inducible T Cell Targeting of Cancer Cells. Mol. Ther. 24: S110.
CrossRef
43. Kudo K, Imai C, Lorenzini P et al. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res. 2014; 74(1): 93–103.
CrossRef
44. Davila E, Tamada K, inventors; University Of Maryland, Baltimore, assignee. Universal anti-tag chimeric antigen receptor-expressing t cells and methods of treating cancer, 2012.
45. Urbanska K, Lanitis E, Poussin M et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012; 72(7): 1844–52.
CrossRef
46. Ma JS, Kim JY, Kazane SA et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc. Natl Acad. Sci. USA 2016; 113(4): E450–8.
CrossRef
47. Cao Y, Rodgers DT, Du J et al. Design of Switchable Chimeric Antigen Receptor T Cells Targeting Breast Cancer. Angew Chem. Int. Ed. Engl. 2016; 55(26): 7520–4.
CrossRef
48. Rodgers DT, Mazagova M, Hampton EN et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl Acad. Sci. USA 2016; 113(4): E459–68.
CrossRef
49. June CH. Remote Controlled CARs: Towards a Safer Therapy for Leukemia. Cancer Immunol. Res. 2016; 4(8): 643.
CrossRef
50. Siegler EL, Wang P. Preclinical Models in Chimeric Antigen Receptor-Engineered T-Cell Therapy. Hum. Gene Ther. 2018.
CrossRef
51. Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 2017; 7(1): 10541.
CrossRef
52. Taraseviciute A, Kean L, Jensen MC. Creation of the First Non-Human Primate Model That Faithfully Recapitulates Chimeric Antigen Receptor (CAR) T Cell-Mediated Cytokine Release Syndrome (CRS) and Neurologic Toxicity Following B Cell-Directed CAR-T Cell Therapy. Blood 2016; 128(22): 651.
53. Yang Y, Jacoby E, Fry TJ. Challenges and opportunities of allogeneic donor-derived CAR T cells. Curr. Opin. Hematol. 2015; 22(6): 509–15.
CrossRef
Affiliations
Eduardo Laborda & Travis S Young*
The California Institute for Biomedical Research, CA 92037, USA.
* Author for correspondence: tyoung@calibr.org
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</