Making the grade: untangling the myths of raw materials used for the manufacture of cell- & gene-based medicinal products
Cell Gene Therapy Insights 2018; 4(3), 207-225.
10.18609/cgti.2018.022
Cell and gene therapy medicinal products (CGP), like other medicines for human use, are expected to be consistently manufactured to a defined quality. That quality is demonstrated through preclinical and clinical studies to be suitable based on an overall assessment of the risks (including quality) and benefits of the product when used to treat a medical condition. Achieving a consistent quality product requires an overall manufacturing control strategy including control of materials, control of the process, control of any intermediates, drug substance and final drug product. Consequently the quality of materials, including raw materials, used to manufacture are paramount to the final CGP quality. This review aims to cut through various mythologies around raw materials by taking a regulatory science approach to discuss raw materials selection.
Cell and gene therapy medicinal products (CGP), like other medicines for human use, are expected to be consistently manufactured to a defined quality. That quality is demonstrated through preclinical and clinical studies to be suitable based on an overall assessment of the risks (including quality) and benefits of the product when used to treat a medical condition. Achieving a consistent quality product requires a manufacturing process that is well controlled. Consequently an overall control strategy is necessary [1–3], this will include control of materials, control of the process, control of any intermediates and the bulk active substance (drug substance) and final drug product. Key to understanding how to implement a control strategy is the understanding that release testing only confirms quality, it cannot alter quality. Product quality is defined by the manufacturing process itself, and how well it controls quality and allowable variation in quality. From here it is clear that the quality of the materials used to manufacture are paramount to the final CGP quality.
Here we discuss raw material-related issues facing developers of those cell, gene and tissue products (CGP) that are regulated as medicinal products for human use (also variably termed biologics, pharmaceuticals, drugs), collectively termed advanced therapies medicinal products in the EU. These products require for example a Biologics License Application in the USA or a Marketing Authorization Application in the EU before marketing. The discussion in this paper is intended to be general regulatory science and not specific to any particular country. These regulatory principles are agreed though the International Council for Harmonisation (ICH) and also followed in World Health Organisation (WHO) guidelines. Where possible examples and references from ICH and WHO are used; these are supplemented with some specific examples from the experience of the authors. Other useful references covering raw materials [4,5] and associated considerations are identified throughout. It is the responsibility of the product developer (used here to mean the current or eventual holder of the license to market) to comply with any additional country-specific requirements.
This discussion is not intended to include cell and tissue transplantation, but many of the same considerations apply, although the regulatory environment for transplantation will differ from that described here. Starting materials such as human cells and tissues, or cell substrates and plasmids for vector manufacture and excipients are not within the scope of this review. However, the same general principles will apply in addition to other considerations. As this is a discussion of regulatory science, it is applicable to all stages of development, and risk assessments will take into account the stage of development.
Terminology
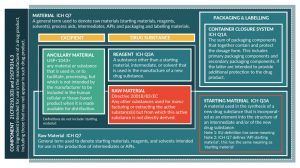
Before moving to the regulatory science, it is necessary to begin by addressing terminology because there are various terms in common use that are defined differently by different institutions. For example, the term ‘raw material’ is preferred here, yet this term is defined differently in EU pharmaceutical legislation and the EU good manufacturing practices (GMP) rules (Figure 1). Here, we favor the ICH terminology because their terms have already been internationally accepted and are applied across the pharmaceutical industry, including biotechnology. The use of ICH terminology in technical documents such as the ICH common technical document (CTD) [6] and reports means these documents can be shared with regulators in most countries without risk the terminology used will be a hindrance to review.
As can be seen from Figure 1, a variety of terms exist to describe categories of materials used in the manufacture of medicines (and a more comprehensive glossary is provided in Supplementary Table 1 and [7]). Within regulatory texts a range of other terms can be used, such as reagents, processing aids, solvents, buffers and so forth, often in combination with the term raw materials. The US Pharmacopoeia (USP) introduced a new term specifically for CGP in general chapter Ancillary materials for cell, gene, and tissue-engineered products [8,9]. While ‘ancillary materials’ is broadly synonymous with ‘raw materials’, the term can encompass for example tissue culture flasks, vessels, transfer bags and tubing sets. In the authors’ opinion, these could also be considered raw materials where they are critical manufacturing materials, such as tissue culture flasks used for adherent cell culture. Similarly, for gene therapy vector manufacture and protein therapeutics, chromatography resins are critical materials for manufacture. The use of single-use culture systems is also increasingly commonplace in biotech manufacturing. Whether these materials meet the definition of raw materials or not, information on the quality of all critical materials must be presented and justified to regulators within section 3.2.S.2.3 of the CTD. Since consideration for containers, culture vessels, chromatography resins and similar items is somewhat different, these will not be discussed here.
A plethora of additional terminology is used by suppliers of raw materials, these need to be treated with caution because standards terms have not been agreed. In many cases, the terms are clearly created for marketing purposes rather than to be meaningfully informative, for example ATMP ready, serum-free, chemically-defined. While these labels may be helpful to create a short-list of possible sources for raw materials, they should not be considered a short-cut to qualifying any source. A commonly misused term that needs to be addressed is ‘GMP-grade’. A grade is a quality standard, i.e., defined by a specification consisting of a list of tests, references to analytical procedures, and appropriate acceptance criteria that are numerical limits, ranges, or other criteria for the test described [10]. By contrast, GMP is a quality system, which is the sum of all aspects of a system that implements a quality policy and ensures that quality objectives are met [10]. For medicines, the generally acceptable grade of commonly used raw materials is provided in the appropriate pharmacopoeia monograph, although some uses of the raw material may on occasion require a different specification.
The main focus of this article is to address sourcing of raw materials for which there is no pharmacopoeia monograph, and includes materials for which there may be a general chapter or other guidance.
Quality standards of raw materials
To avoid possible confusion with the terms ‘quality attribute’ and ‘critical quality attribute’ as applies to active substances and drug products, the term ‘critical materials attributes’ (CMA) will be used here. The term CMA is commonly used by pharmaceutical companies including biotechs (though as yet does not appear in ICH guidelines), and is synonymous with critical quality attributes when applied to raw materials. Table 1 lists the general categories of CMA to consider for any material, along with examples for a protein. Control of adventitious agents (infectious contaminants) will be discussed separately from general raw materials quality, although they are both part of quality.
| Table 1: General considerations for the quality of any material | |
|---|---|
| Test category | Description and examples (protein) |
| Identity | Simple test to confirm identity of intended substance, e.g., molecular weight on gel |
| Purity | Purity of intended substance, e.g., ELISA for specific protein reported as % of total protein |
| Product-related impurities | Aggregates, truncated forms (e.g., where inactive), different glycoforms by methods such as HPLC, mass spectrometry. |
| Process-related impurities | Process residuals such as antibiotics, hosts cell proteins/DNA by methods such as ELISA, qPCR. |
| Contaminants/safety | Sterility, endotoxin, mycoplasma, viral testing, but may include other contaminants. |
| Biological function | Method/s to confirm biological activity/ies (raw material) or potency (drug substance/product); e.g.,bioassay |
| Content | Total amount of substance present, e.g. total protein by UV spectrometry. |
| General tests | Tests such as appearance, pH, osmolality, conductance, etc. |
The first consideration for any raw material is whether its quality can be fully defined through testing, that is, can all the CMA be identified? For the most part this is true for small molecule chemical raw materials and this has allowed pharmacopoeias to prepare monographs defining a grade that is generally acceptable for all pharmaceutical uses. However, there may be specific situations where these monographs are not suitable for some reason and need to be adapted, but this is less common. One benefit of defining a pharmacopeia grade (monograph) is this simplifies the regulatory information provided in section 3.2.S.2.3 of the CTD to a simple table of raw materials and their grade [Table 2]. There is no need to provide a specification as long as the raw material is in compliance with the monograph, although it will be necessary to describe any testing undertaken on receipt. For the most part these tests are already defined in the monograph and often the tests applied are also standard pharmacopeia test methods. The second benefit is the ability to substitute the raw material for another source that also complies with the monograph without the need to demonstrate comparability. This also means the supplier can be omitted from relevant sections of the CTD avoiding the need to make changes to the dossier text (facilitating dossier lifecycle management).
| Table 2:Example dossier (3.2.S.2.3) information for chemical raw materials | ||
|---|---|---|
| A. Compendial chemical raw materials | ||
| Raw material | Grade | Process step(s) |
| Carbon dioxide | USP/Ph. Eur. | Steps 2, 3 |
| L-glutamine | USP | Steps 2, 3 |
| Sodium chloride | USP/Ph.Eur./JP | All (wash steps) |
| Water for injections | USP | All |
| B. Example of incoming raw material testing; sodium chloride | ||
| Test | Test method | Acceptance criteria |
| Identification | ||
| Sodium | Monograph | Complies with monograph |
| Chlorides | Monograph | Complies with monograph |
| C. Non-compendial chemical raw materials | ||
| Raw material | Grade | Process step(s) |
| Glucose monohydrate † | In-house | Steps 2, 3 |
| D. Example of in-house specification; glucose monohydrate † | ||
| Test | Test method | Acceptance criteria |
| Appearance | White, odorless, crystalline powder | |
| Identity | ||
| Precipitation reaction | Monograph | Complies with the test |
| Purity | ||
| Clarity of solution | Ph.Eur 2.2.1 | Clear to opalescent |
| Acidity or alkalinity | Ph. Eur. 2.2.3 | Complies with test |
| Heavy metals | Ph. Eur. 2.4.8, method B | Not more than 10 ppm |
| Water content | Ph. Eur.2.5.12 | 4.0–9.5% |
| Assay | ||
| Glucose anhydrate | In-house (titrimetric) | Not less than 99.0% |
| † Glucose will have been extracted from a biological source but it is a small molecule that can be fully characterized. There are monographs for glucose but this is an example of where, for some reason, an in-house grade is defined. | ||
The situation for biological raw materials is more complex because the quality of biological raw materials cannot normally be fully defined. This point is made for example in the European medicines directive [11] within the definition of a biological medicinal product: A biological substance is a substance that is produced by or extracted from a biological source and that needs for its characterization and the determination of its quality a combination of physico-chemical-biological testing, together with the production process and its control. In contrast to chemical raw materials, biological raw materials need both physicochemical testing and biological testing (tests for biological activity). This is because physiochemical tests cannot fully confirm if the biological material has the required activity because they are large molecules subject to complex folding and a variety of post-translational modifications. This heterogeneity can also vary due to genetic differences in the source materials such as cell substrates and individual animals, humans, insects or plants, as well as the conditions used to culture or extract the biological material. These differences can be significant, for example a supplier that produces a recombinant protein for research use may only undertake a crude purification and the material may contain a range of impurities that might be detrimental to a CGP manufacturing process. The same protein may be available as a licensed medicine, in which case it will have few and well-controlled impurities as required for therapeutic use. However, the same therapeutic protein from a different manufacturer, even if biosimilar will have differences in its quality due to differences in the starting materials and manufacturing process. While the two may be therapeutically equivalent this does not guarantee they will give the same performance in vitro in a CGP manufacturing process; although given the high quality they might.
For these reasons monographs for biological materials cannot be completely comprehensive since both process-related impurities and product-related variants will differ due to differences in manufacturing processes. Such monographs are also largely limited to proteins that are used therapeutically. When considering a pharmacopoeia monograph it is important to confirm the purpose of the monograph, or other text, when considering its suitability. Some individual monographs describe the quality of raw materials but others describe excipients, active substances or even drug products. For example Ph. Eur. has general monographs covering topics such as 0784 (Products of recombinant DNA technology) and 2034 (Substances for pharmaceutical use), which become mandatory only where an active substance monograph such as 1316 (erythropoietin concentrated solution) is applied. Some test methods within this monograph do not include acceptance criteria; instead it states the limit is approved by the competent authority. Likewise the equivalent USP monograph for epoetin states: The presence of the impurities, host cell DNA, and host cell protein in epoetin is process-specific, and is controlled through the purification process. The impurity levels are determined by validated methods and limits approved by the competent regulatory authority. Erythropoietin requires a mammalian expression system, yet no adventitious agent testing is mentioned in Ph. Eur. except limits for endotoxin. This is because Ph. Eur. general chapter 0784 (Products of recombinant DNA technology) applies for active substance and that specifies the need for viral safety assessment. However, these monographs could be used as the basis of a specification for erythropoietin as a raw material, but in-house specifications for relevant impurities would need to be established, and other test methods and acceptance criteria might not need to be the same. If erythropoietin was to be used as a raw material for the manufacture of a CGP this would not remove the need to also consider viral safety including cell bank testing [12], and inclusion of validated viral clearance steps [13]. As a result, unlike monographs for chemical materials, it is not possible to merely cite the monograph and claim compliance, some additional test and acceptance criteria are needed. To summarize this discussion on pharmacopoeias, it is important to understand the purpose of an individual monograph (e.g., for USP and Ph. Eur. check what it says under the heading definition) to ensure it is applied correctly when used to define the grade of a raw material.
For the reasons discussed above, in most cases materials of biological origin cannot be substituted without evidence of comparability [14]. Consequently, it will be necessary to define an in-house specification that addresses the CMA relevant to the specific use of the raw material for CGP manufacturing; and this specification will need to be provided and justified in the CTD [Table 3]. Where licensed medicines are used as raw materials it is recommended to include the license number to ensure the product is correctly identified. This also means the supplier must be identified in the CTD, and any change of supplier will require an update to the dosser, including evidence of comparability [14].
| Table 3: Example dossier information for biological raw materials | |||
|---|---|---|---|
| A. Biological raw materials | |||
| Raw material | Grade | Supplier | Process step(s) |
| Collagenase | In-house | Super Enzymes Ltd | Step 1 |
| Fetal bovine serum (FBS) | In-house | Sera Supplies Inc. | Steps 2, 3 |
| Porcine trypsin | In-house | Super Enzymes Ltd | Steps 3, 4 |
| B. Example of incoming raw material testing; FBS | |||
| Test | Test method | Acceptance criteria | Test |
| Identification | |||
| Identity | In-house (electrophoresis) | Complies with reference | Identity |
| Assay (biological activity) | |||
| Growth test | In-house (population doubling time, PDT) | PDT, 24–36 hours | Growth test |
| C. Specification, FBS | |||
| Test | Test method | Acceptance criteria | |
| Identity | |||
| Identity | In-house (electrophoresis) | Complies with reference | |
| General tests | |||
| Osmolality | Ph.Eur. 2.2.35 | 280–365 mosmol/kg | |
| Total protein | Ph. Eur. 2.5.33 | 30–45 mg/mL | |
| Impurities | |||
| Hemoglobin | In-house (spectrophotometry) | <4 mg/mL | |
| Contaminants | |||
| Endotoxins | Ph. Eur. 2.6.14 (LAL method) | <10 IU/mL | |
| Sterility | Ph. Eur. 2.6.1 | No growth | |
| Mycoplasmas | Ph. Eur. 2.6.7 | No growth | |
| Bovine viruses † | In vitro diagnostic test kits | Not detected | |
| Assay (biological activity) | |||
| Growth test | In-house (population doubling time, PDT) | PDT, 24–36 hours | |
| † Not listed here to simplify table. Further details of control of adventitious agents are also included in 3.2.A.2 of the CTD. | |||
Any in-house test methods will also need to be described in 3.2.S.2.3 of the CTD, and should be qualified (shown to be fit for purpose) from the outset, and validated prior to marketing approval. Where suitable, compendial test methods can be used, these do not need to be described or validated beyond what is described in the text. Where suitable, the use of licensed in vitro diagnostic tests (e.g., for viral testing) will also avoid the need to develop and validate methods and therefore simplify the dossier text. A suitable method, or methods, to measure the biological activity of biological raw materials will in most cases need to be developed and validated, even if the supplier includes an activity test. For example, collagenases tend to have more than one enzyme activity, and there are multiple test methods available, ranging from artificial peptides to mouse foot pad. Whether any of these methods measure an appropriate activity when used to digest a particular tissue biopsy needs to be established. In this case, it may be necessary to develop an in-house assay that for example measures the rate of digest of a model tissue. By contrast, trypsin activity is more specific and can be reliably determined by comparing the rate of hydrolysis of benzoylarginine ethyl ester hydrochloride to a trypsin reference material (trypsin BPR) [15].
For most biological raw materials the specification provided in 3.2.S.2.3 of the CTD will need to be supported by details of the adventitious agent risk assessment and control measures that should be described and justified in 3.2.A.2 of the CTD. Adventitious agents are discussed below.
Some raw materials, such as antibodies or cytokines, may be intended as reagents for medical devices, or medical devices in their own right (e.g., have CE-mark in EU). It should not be assumed these materials are suitable for the manufacture of CGP, as their approval is only valid and meaningful in the context of the medical device authorization, and they may be intended only for in vitro use. It is therefore recommended to undertake the same due diligence on these as for other raw materials.
Identifying a supplier that can meet the desired quality is not sufficient. The ability of the supplier to produce a raw material of consistent quality also must be considered. Assuming the specification is suitably comprehensive, the allowable ranges should give a good idea of the expected variability of each CMA. The use of a suitable quality system (see below) should also ensure this specification can be consistently met by the manufacturer. Where the raw material can be fully characterized, there may be limited need to confirm batch-to-batch consistency, deepening on how tight the suppliers’ specification is. For raw materials of biological origin the specification is likely to have wider ranges and the CGP developer will need to understand if the allowable variation is acceptable or whether they need to work with the supplier to achieve a tighter specification. As discussed above, the relevant biological activity of the raw material may need to be assessed by the CGP developer, and consequently may not be well controlled by the supplier either because they don’t test for biological activity, or because the test they apply isn’t meaningful or relevant for the CGP developers’ use of the raw material. In these situations, there will be a greater need for the CGP developer to explore batch-to-batch variability, for example ICH Q7 [10] recommends at least three batches are assessed.
Finally the developer needs to understand the stability of the raw material, both as provided by the supplier and where diluted or combined with other raw materials to prepare buffers, culture media and so forth. For small molecule chemical raw materials there will be extensive literature. For more complex materials, especially biological materials, it will likely be necessary for the developer to undertake in-house studies to confirm the shelf-life. Likewise the stability of solutions prepared in-house such as buffers and culture media will need to be confirmed. In these situations the same general principles described in ICH Q5C [16] should be followed and adapted as necessary.
Adventitious agents
Infectious adventitious agents can be contaminants in raw materials of biological origin if not properly controlled during manufacture and handling of the raw material. Since CGP products cannot normally be subject to viral clearance, inactivation steps or sterilization there is a greater need to control the risk of introduction of adventitious agents through raw materials. Confounding this, compared to biotech, a wider array of biological raw materials are used in the manufacture of CGP, including but not limited to serum, enzymes, monoclonal antibodies, cytokines and growth factors. These raw materials derived from bacteria, plants, humans and other mammals, and sometimes insects, are used as serum and as expression systems for recombinant proteins. Consequently, each brings unique risks and differing possibilities to control those risks [Table 4, column 2].
These risks make it essential to understand the source and origin (including country of origin) of each raw material, not merely the supplier but the original manufacturer of the raw material (where different from supplier). This also requires some understanding of the raw materials’ manufacturing process; the more complex the material, the more details will be needed. This can lead to conflicts with the supplier who may have intellectual property as well as other commercial interests to protect. Some regulatory agencies will have a master file system, most notably the US FDA allow a wide range of materials to utilize their drug master file (DMF) system [16]. By contrast in the EU, the European active substance master file (ASMF) can only be used for small molecule active substances [17], no system is available for biological active substances or raw materials. Even where a master file system is possible, the responsibility for the CGT product safety still lies with the CGP developer; it isn’t an exemption from the responsibility to understand the quality of the materials being used. Furthermore, the master file may not be reviewed by the regulator until it is cross referenced by a user, as is the case with the FDA; so unless it has been used by others there may be a risk the regulator considers it unacceptable. For these reasons it is recommended to ask the same questions of the supplier whether a master file system is available or not, and not accept any responses that state this information is in the master file so will not be disclosed.
Since CGP products are themselves mostly parenteral products, they need to be sterile and consequently are manufactured under sterile conditions. This requires all raw materials either be sterilized before addition, considering also the possible impact of any bioburden during storage, or sourced as sterile. How raw materials are rendered sterile is also a consideration as this can also impact the final quality. This need for aseptic manufacturing of some raw materials should not be confused with the use of a quality system, such as GMP (discussed later). GMP can be applied to both aseptic and non-aseptic manufacturing settings, so GMP in itself doesn’t ensure sterility; rather, it is the design of the manufacturing facilities used that is most pertinent. In most cases, microbial control of raw materials is straightforward; most can at least be subject to validated sterile filtration.
The more complex risk to understand is that of viral contamination as this requires significant information from the supplier. For example, a recombinant protein could pose a similar risk to a native extracted protein if it is derived from a mammalian expression system. In that situation, it would be necessary to establish whether the cell banking system used was suitably tested [12], whether biological materials such a fetal bovine serum are used for culture, and whether viral clearance steps are applied downstream [13]. By contrast, for a recombinant protein of microbial origin the cell substrate poses no obvious viral risk; however, the culture broth could be of animal origin. This very issue occurred with a collagenase used in islet transplantation. The collagenase was purified from culture supernatants of Clostridium histiolyticum grown in broth prepared from bovine brain and porcine heart of uncertain origin, though thought to be of US origin [19]. In addition to the unknown viral risk, bovine brain is also a high-risk material for transmissible spongiform encephalopathies (TSE), as defined in the World Health Organization (WHO) risk tables [20].
Collagenase is a good example of the need to understand not just the quality of the raw material but also its origin and, to some extent, the quality of the materials used to prepare it. Determining the appropriate level of detail requires a good general understanding of the sorts of materials used and their origin. For example, a supplier may provide a culture medium they claim to be animal component-free (or similar nonstandard term), and the composition is likely to include amino acids, some of which are commonly derived from a variety of animal sources such as hair, hide or skins by hydrolysis. While these pose no obvious risk for adventitious agents due to the processes used, they are animal-derived. To ensure TSE risks are mitigated, exposure of the material to a pH of 1 to 2, followed by a pH of > 11, followed by heat treatment at 140 °C for 30 minutes at 3 bar [21] is required. Glycerol, fatty acids and fatty acid esters have a variety of uses, including the production of plastics, and are commonly prepared from animal tallow (fat) [Table 4]. As long as appropriately rigorous production processes are utilized, these materials pose negligible or no risk for TSE or virus transmission [20,22,23]; but this needs to be confirmed. A common example of their use is for the plastic parts of container closure systems and typically the developer requests confirmation from the supplier that these comply with procedures that minimize TSE risk. Lactose and casein are extracted from whey (left-over liquid from cheese production) following coagulation with rennet (although alternatives exist). Rennet is an extract from ruminant abomasum, which may be bovine. As long as no other ruminant materials, with the exception of calf rennet, are used in the preparation of such derivatives (e.g., pancreatic enzyme digests of casein) these are considered acceptable [21], so long as the milk was also fit for human consumption. For all these materials there is also a need to confirm that measures are in place to avoid cross-contamination with other materials manufactured in the same facilities, both to control the possible spread of adventitious agents, but also to avoid other substances contaminating the material.
| Table 4: Example considerations for different sources of raw materials | ||
|---|---|---|
| Example raw material | Example adventitious safety considerations* | Example safety concern/s for process-related impurities* |
| Chemical | ||
| Antibiotics, especially beta lactams | Usually none; however use may obfuscate low-level microbial contamination if used in manufacturing. | Hypersensitivity or allergic reactions, especially beta lactams (should be avoided). |
| Dimethyl sulfoxide (DMSO) | Usually none. | Class 3 solvent [27]; solvents with low toxic potential. Class 3 solvents have a permitted daily exposure of 50 mg or more per day. Potential for leachates from container closure system. Known to lead to hypersensitivity reactions depending on route of administration and overall exposure [28, 29] can include central nervous system toxicity [30]. |
| Glycerol (tallow-derivative) | Usually none so long as prepared by either 1) trans‐esterification or hydrolysis at not less than 200 °C for not less than 20 min under pressure, or 2) saponification with sodium hydroxide solution, at a concentration of 12 mol/l ≥ 95 °C for >3h or ≥140 °C for ≥ 8 min, or 3) distillation at 200 °C [23] | Generally none. |
| Microbial Origin | ||
| Collagenase | Check fermentation broth or other manufacturing materials are not animal-derived. Check control of sterility and endotoxin. | May cause hypersensitivity or allergic reactions. |
| Recombinant protein, E.coli- derived | Check fermentation broth or other manufacturing materials are not animal-derived. Check control of sterility and endotoxin. | May cause hypersensitivity or allergic reactions. |
| Plant origin | ||
| Plant trypsin | Confirm procedures in place to control exposure of source plants to wild-life and suitable surface decontamination of collected plants. Confirm microbial control and test for spiroplasmas. | May cause hypersensitivity or allergic reactions. |
| Mammalian origin | ||
| Porcine trypsin | Confirm country of origin and suitable viral testing of donor animals. Confirm processing includes validated viral clearance step/s and sterilisation step. Consider irradiation to reduce risk of porcine circovirus [31, 32]. | May cause hypersensitivity or allergic reactions. |
| FBS | Confirm country of origin and suitable viral testing of donor animals. TSE risk assessment [20, 22, 23, 26]. Confirm processing includes validated viral clearance step/s such as gamma irradiation, nanofiltration (25, 33). | Hypersensitivity [34], anaphylaxis [35], undetected virus [36] |
| Recombinant protein, CHO-derived | Confirm suitable testing of cell banking system [13]. Confirm inclusion of validated viral clearance step/s [13] and suitable microbial control. | May cause hypersensitivity or allergic reactions. |
| Collagen | Country of origin should have minimal TSE risk; animals should be fit for human consumption. Bones pose a theoretically higher risk than hides, where bones are used skulls and spinal cords should be removed [21, 23]. | None normally. |
| Casein (milk-derived) | TSE risk is minimal [21, 23], animals should be fit for human consumption. | None normally. |
| Insect Origin | ||
| Insect cell-derived | Test for spiroplasmas and Insect viruses | May cause hypersensitivity or allergic reactions. |
| Baculovirus (where used to transduce insect cells) | Not known to cause human disease but can infect human cells. Viral clearance steps designed to specifically remove baculovirus should be used. | Potential carry over of infectious baculovirus. Where used as a vector, potential to transfer unintended genetic material. |
| * These are example considerations only and not meant to be comprehensive. | ||
Serum is commonly used and so merits some additional comment. It is expected that some form of viral inactivation be applied to serum, e.g., human, bovine; for example ≥30 kGy of γ-irradiation is typically used for bovine serum [24–26]. There is a misunderstanding that the adventitious agent risk of human serum or serum-derived materials such as human platelet lysate are significantly less than equivalent serum-derived materials from animals. Both pose similar but qualitatively different risks and so could be equally acceptable so long as appropriate risk mitigation procedures are applied [24]. For example, human platelet lysate can be prepared from a pool of donor platelet rich plasma in compliance with the rules for blood, which includes testing of donors, questionnaires, testing of pooled plasma and so forth. The greater the number of donors, the greater the risk becomes that the pool contains virus, despite these controls. In comparison, a similar number of bovine donors might be collected to create a bulk pool of serum; similarly there would be controls and testing in place. Given bovine viruses are less likely to be infectious to humans, would it be fair to say bovine serum poses a greater risk? Indeed, many CGP developers use human serum, plasma, or platelet lysates without additional viral clearance (e.g., nanofiltration) or inactivation steps (e.g., gamma irradiation), so it could be said the risks are better mitigated. For human plasma-based medicinal products the number of donations can be very large and it is therefore necessary to include viral clearance or viral inactivation steps. Typically, at least two steps are required, each of which achieve their effects differently, e.g., irradiation, nanofiltration, detergent, chromatography. This is necessary because different types of virus have different susceptibilities to each approach.
In order to undertake a risk assessment for each raw material the developer therefore needs internal expertise in materials and how they are typically prepared, especially materials isolated from biological sources. With some materials used to manufacture a raw material it may be necessary to ask further questions on their quality and method of manufacture. Details of the production process of the raw material itself will need to be understood, especially where the materials used pose a risk for transmission of adventitious agents. Whether a potential or real risk for adventitious agents, if a risk is identified the developer needs to ask how the raw material manufacturer mitigates these risks, e.g., are there viral clearance or inactivation steps included. Only then can the developer prepare an appropriate questionnaire for the supplier; although the answers may trigger further rounds of questions. Where the supplier has not sufficiently mitigated risks, the source may still be suitable if the developer can implement further steps before use, such as irradiation or nanofiltration.
Process-related impurities risks
In addition to their adventitious agent risks, there are risks associated with residual raw materials (process-related impurities) if not suitability controlled [Table 4, column 3]. A few raw materials may have unacceptable risks for most processes, such as beta-lactam antibiotics, which are generally discouraged. After considering the possible risks of residual raw materials, the next consideration is whether these risks can be sufficiently mitigated. Considerations here include, the quantity added to the process, how well the process clears the impurity, how much is detected in the final drug product, the route of administration, dose (e.g., volume administered) and frequency of administration (posology), patient population (e.g. paediatric, adult, immunocompromised), co-medication and likely other considerations.. Assuming the raw material cannot be substituted for a less toxic raw material, this assessment will also provide a target value for purification steps, remembering the general regulatory principle that impurities should be reduced as far as practical, even if they are not toxic.
Initial estimates of process-related impurities may be based on theoretical calculations but these estimates will need to be verified through testing. In some cases, test methods for process-related impurities may not be sufficiently sensitive and the impurity may be lower than the limit of quantitation and even the limit of detection of the method. It is generally expected the steps that contribute to clearance of process-related impurities are identified, and where the impurity can’t be measured in the drug product it may be necessary to test in-process at a point where it is measureable. Where the impurity poses a risk, spiking studies can be undertaken on the step(s) that contribute to clearance to evaluate their capacity or ideally overcapacity to remove the impurity. In some cases, it will be possible to omit testing for the impurity where purification steps have been shown through validation to have overcapacity to remove the impurity. For marketing approval these quality data are then supported by clinical safety data from clinical trials; remembering some adverse events may be rare and so not observed until widespread clinical use.
Quality systems
In general terms, pharmaceutical GMP aims to ensure medicines are consistently produced to defined quality standards, those being the product-specific specifications defined by the manufacturer. By contrast, quality is the degree to which a set of inherent properties of a material fulfil the requirements [1]; these requirements are defined by the user (developer) in the form of a specification. A specification is a list of tests, references to analytical procedures, and appropriate acceptance criteria that are numerical limits, ranges, or other criteria for the test described. It establishes the set of criteria to which a material should conform to be considered acceptable for its intended use [10]. For medicines, by the time the product is approved for marketing, these standards have been clinically qualified [37,38]; that is, demonstrated to have an acceptable risk/benefit for a particular therapeutic use.
Other industry sectors also use the term ‘good manufacturing practice’ but their objectives can differ. Importantly, regulatory agencies do not audit or license manufacturing sites for raw materials, nor are there any legal requirements for raw materials to be manufactured within a particular quality system. Consequently any claim of GMP compliance is self-certified by the supplier. This raises various questions such as the type of ‘good manufacturing practices’ the supplier is using, whether the overall quality system in place is suitable for raw material manufacturing, how is GMP compliance confirmed, are any identified deficiencies addressed in a timely manner, and so forth. In the absence of an official GMP license, it is left to the CGP developer to audit the supplier and confirm the quality system is suitable. It should also be remembered that pharmaceutical GMP guidelines are not prescriptive but describe general principles that might be satisfied in a number of ways. This allows a medicine developer to define the quality of their product, considering the intended use (i.e., to treat a particular disease). Likewise, the manufacturer of a raw material will define the quality they wish to achieve, for example this could be a material with a purity of 99.9%, or they could decide a purity of 90% is acceptable for their customers perhaps because achieving higher purity would significantly increase costs. When determining the desired grade, a supplier might chose to meet a defined quality standard such as a pharmacopoeia, or they may define their own standards. That standard may or may not include control of adventitious agents, depending on whether the intended market is perceived to want the material sterile and whether viral safety is relevant. Unless the supplier fully understands the needs of a customer, the material they supply may not be suitable; furthermore each customer may have differing quality requirements. It is the overall risk assessment of the raw material based on its quality (specification) and its suitability for the CGP developer’s particular needs that should be considered. Unfortunately there are no short-cuts for non-compendial raw materials.
A situation where the underlying quality system will not need to be audited is where the raw material of interest is already a licensed medicine or medical device, since their ongoing marketing approval will require compliance. However, this doesn’t preclude the need to consider quality, for example the medicine may be for oral not parenteral use meaning it may not be sterile, it may instead have a defined level of bioburden.
Another approach to sourcing raw materials is to manufacture them in-house or use a subcontractor to manufacture them. This is unlikely to be desirable unless the material is a critical material that isn’t available, such as a starting material. For example, manufacturers of genetically modified cell products will manufacture or subcontract manufacture of the viral vector. Dendreon used a contract manufacturer to supply their recombinant fusion protein consisting of prostatic acid phosphatase (PAP) fused to GM-CSF, used in the manufacture of Provenge [39].
Discussion & conclusions
Control of raw materials forms part of the overall control strategy for any medicinal product [1,2]. Raw materials for CGP take on greater importance, however, as CGP are inherently more complex and consequently use more complex raw materials in their production. Poor control of raw materials will lead to poor control of CGP quality, will likely contribute to product variability, and may impact safety. Because the developer is ultimately responsible for the safety of their products, it is imperative that the CGP developer fully understand the origin and quality of all raw materials used. To achieve the required depth of understanding of the quality of each raw material requires open and honest dialogue with suppliers. From a regulatory science perspective, the use of drug master files to protect the supplier’s intellectual property is in conflict with the needs of the developer to ensure the quality and safety of their products. The use of drug master files therefore is a barrier to the CGP developer’s ability to thoroughly assess risk, and negotiations with the supplier may be necessary to ensure all relevant information are disclosed.
Terminology is sometimes a barrier to understanding raw material quality. A variety of terms are used by suppliers to describe materials used in manufacturing therapeutic products, but standard terms have not been established, and much of the terminology in use appears to have been created primarily for marketing purposes. CGP developers should bear this in mind when sourcing raw materials.
Risk assessments for raw materials are an essential part of CGP development, but to assess risk appropriately the developer must have the expertise to understand sources and magnitude of risks. Even simple, chemically defined raw materials may be derived from biological sources such as amino acids, fatty esters and sugars, some of which may add risk. For many biological-origin raw materials, extraction and purification steps are sufficient to render risks minimal. In some cases, however, risk considerations are more complex. Materials derived from ruminants, for example, present risks associated with TSE. Neutralizing TSE infectivity, however, requires harsh conditions that may be destructive to the raw material. Consequently, risk mitigation for ruminant-derived materials requires special attention to the collection of the source materials [21].
As a general regulatory principle, both testing and mitigation procedures are necessary for many raw materials of biological origin. For example, where a protein is manufactured using a mammalian expression system, the cell substrate is thoroughly tested for adventitious agents and downstream purification should include two orthogonal approaches to reduce viral risk. Any risk assessment undertaken by the CGP developer will only be as good as the knowledge available, and assumes suitable expertise of those undertaking the assessment. As knowledge and experience evolve, such risk assessments should be updated. Continuous learning and improvement should be central principles for any developer.
Risk assessments of raw materials should also consider the risk posed by residual raw materials that will inevitably remain at some level in the final CGP drug product as process-related impurities. These risks will inform aspects of process design that lead to downstream removal of the raw material. In most cases where process-related impurities pose a safety concern there may not be less toxic alternatives, and so additional clearance steps might be necessary. Regulatory principles dictate that all impurities should be identified, and reduced as far as possible even if not toxic. If a process-related impurity poses a risk it will need to be routinely tested. As these impurities are not always measurable in the final drug product, such testing may also need to be carried out in-process. Where the risk is minimal, testing may be omitted entirely if the ability to clear the product-related impurity to sufficiently low levels is demonstrated through validation.
The remaining aspect is that of the use of a suitably quality system to ensure processes are executed correctly and documented properly, and that the raw material is consistently manufactured to a defined quality. Here, it is essential to understand the difference between a quality system and quality, to avoid the common but mistaken belief that GMP is a grade. Furthermore, good manufacturing practices are defined differently for different sectors, and since raw materials suppliers cannot be licensed by medicines agencies, any claim of compliance with pharmaceutical GMP is self-certified. The developer therefore must audit the supplier to confirm their interpretation of GMP is appropriate and applied correctly. As GMP is a quality system, merely operating that system doesn’t ensure a raw material of suitable quality. The quality of each raw material is defined by the supplier, with or without input from customers, but may or may not be suitable for a specific application by a CGP developer. In some cases, it may be necessary for the CGP to work with the supplier to achieve the desired quality; alternatively, in some cases it may be preferable to have a raw material custom-manufactured under contract.
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
ACKNOWLEDGEMENTS
The authors are deeply grateful to Karin Hoogendoorn for her detailed comments during the drafting of this manuscript.
References
1. ICH. Pharmaceutical development Q8(R2), 2009: Website
2. ICH. Quality risk management Q9, 2005: Website
3. ICH. Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) Q11, 2012: Website
4. BSI. PAS157:2015 Evaluation of materials of biological origin used in the production of cell-based medicinal products – Guide. <a href=”http://shop.bsigroup.com/forms/PASs/PAS-1572015/: BSI Standards Limited; 2015.
5. EBE. EBE concept paper: Management and control of raw materials used in the manufacture of biological medicinal products 2017 Accessed March 2018. :[25 p.]. Available from: Website
6. ICH. The common technical document for the registration of pharmaceuticals for human use: quality – M4Q(R1), 2016: Website
7. BSi. PAS 84:2008 Regenerative medicine. Glossary: http://www.bsigroup.com/en/sectorsandservices/Forms/PAS-84/ [Updated 4/30/2008: Website]
8. Ancillary materials for cell, gene, and tissue-engineered products. USP41-NF36 ed. Rockville, MD: United States Pharmacopeial Convention; 2017.
9. In-process revision: Ancillary materials for cell, gene, and tissue-engineered products. Pharmacopeial Forum. 2017; 43(4).
10. ICH. Good manufacturing practice guide for active pharmaceutical ingredients, Q7, 2000: Website
11. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal of the European Communities. 2001;L 311:0067-128.
12. ICH. Derivation and characterisation of cell substrates used for production of biotechnological/biological products Q5D, 1997: Website
13. ICH. Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin Q5A(R1), 1999: Website
14. ICH. Comparability of biotechnological/biological products: subject to changes in their manufacturing process Q5E, 2004: Website
15. 0694 Trypsin. 9.0 ed. European Pharmacopoeia: Council of Europe; 2017.
16. ICH. Quality of biotechnological products: stability testing of biotechnological/biological products Q5C.1995 11 March 2017. Available from: Website
17. FDA. Drug Master Files: Guidelines: Website
18. CHMP. Guideline on Active Substance Master File Procedure, EMA, 2013: <a href=”http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129994.pdf
19. Caballero-Corbalan J, Brandhorst H, Asif S et al. Mammalian tissue-free liberase: a new GMP-graded enzyme blend for human islet isolation. Transplantation 2010; 90(3): 332–3.
CrossRef
20. WHO. WHO Tables on tissue infectivity distribution in transmissible spongiform encephalopathies, 2010: Website
21. Commission E. Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products (EMA/410/01 rev.3). Official J. Eur. Commun. 2011; C 73: 1–18.
22. WHO. WHO guidelines on transmissible spongiform encephalopathies in relation to biological and pharmaceutical products, 2003: Website
23. WHO. WHO guidelines on tissue infectivity distribution in transmissible spongiform encephalopathies, 2006: Website
24. Reinhardt J, Stühler A, Blümel J. Safety of bovine sera for production of mesenchymal stem cells for therapeutic use. Hum. Gene Ther. 2011; 22(6): 775; author reply 6.
CrossRef
25. Bovine serum. USP41-NF36 ed. Rockville, MD: United States Pharmacopeial Convention; 2017.
26. CHMP. Guideline on the use of bovine serum in the manufacture of human biological medicinal products, EMA, 2013: Website
27. ICH. Impurities: guideline for residual solvents Q3C(R6), 2016: <a href=”http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3C/Q3C_R6__Step_4.pdf
28. Morris C, de Wreede L, Scholten M et al. Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. Transfusion 2014; 54(10): 2514–22.
CrossRef
29. Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014; 49(4): 469–76.
CrossRef
30. Hanslick JL, Lau K, Noguchi KK et al. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol. Dis. 2009; 34(1): 1–10.
CrossRef
31. Petricciani J, Sheets R, Griffiths E, Knezevic I. Adventitious agents in viral vaccines: lessons learned from 4 case studies. Biologicals 2014; 42(5): 223–36.
CrossRef
32. CHMP. Guideline on the use of porcine trypsin used in the manufacture of human biological medicinal product, 2014: Website
33. 2262 Bovine serum. 9.0 ed. European Pharmacopoeia: Council of Europe; 2017.
34. Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood 1997; 89(3): 776–9.
35. Mackensen A, Dräger R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol. Immunother. 2000; 49(3): 152–6.
Website
36. Schupbach J. Induction/activation and detection of occult viral agents present in mammalian cells. Dev. Biol. (Basel) 2001; 106: 425–37; discussion 65–75.
37. CHMP. Report on the expert workshop on setting specifications for biotech products, 2012: Website
38. EBE. EBE concept paper: Considerations in setting specifications 2013 Accessed March 2018. :[26 p.]. Available from: Website
39. CHMP. Provenge (sipuleucel-T). European public assessment report, EMA, 2013: Website
SUPPLEMENTARY TABLE
Supplementary Table 1
Bravery et al Online supplement, Table 1
AFFILIATIONS
Christopher Bravery1*, Sarah Robinson1 & Scott R Burger2
1 Consulting on Advanced Biologicals Ltd, London, UK.
2 Advanced Cell & Gene Therapy, LLC, Chapel Hill, NC, USA.
* Corresponding author: cbravery@advbiols.com
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</