Innovation in Management as a Tool for Accelerated Translation of Advanced Therapies
Cell Gene Therapy Insights 2018; 4(3), 157-172.
10.18609/cgti.2018.011
The manufacture, delivery and administration of advanced therapies (cell-, tissue- and gene-based treatments) comprise a sensitive chain of custody that may be thought of as an integrated service system. The system is challenging to assemble for the predominantly small businesses that seek to compete in this sector. The current investment climate is not favorable to proposals unless they have a high probability of success or are capital-efficient or both. Business models based around the extended enterprise, the collaborative enterprise and the franchise models are relatively under-explored for the sector. This paper sets out the main characteristics of these models and the reasons that they may increase the pace of innovation: by reducing investment burden, by encouraging co-ownership of the chain of custody and by allowing early data capture to assist with investment proposals.
Managing manufacture for new advanced therapies
Innovation is at its most efficient when it conserves the important achievements of the past and modifies them or builds on them to accommodate new insights and new solutions. This process of optimization can be likened to the progressive ascent of optima in a solution space. The peak of adaptation is gradually approached and what was once unusual becomes the commonplace. Management systems assume that this way is how it should be done and it is relatively unusual to find the management system introducing its own radical change. ‘Disruptive technology’ is technology that necessitates a change in ways of working because the old operational model no longer fits [1]. However, disruptive technology is usually the product or the service enabled by that product. The ways of working change to meet the clear and present challenge. By contrast, when an organization is focused on a particular product and a non-obvious disruption to the current ways of working is required to attain it, the innovator will find that the investment of time and money that has proved worthwhile to date in a particular enterprise can often result in a reluctance to disturb the status quo [2,3].
The purpose of this paper is to highlight a disruptive approach to innovation in manufacturing for advanced therapeutic products that may offer advantages over current ways of working. It has not so far been put to the test in practice (although some organizations offer signposts to this, see below) but has withstood many conversations and criticisms from specialists in a variety of backgrounds. It is offered here for consideration as a potential alternative to accepted approaches to translating advanced therapy innovations to commercialization.
Innovation in advanced therapies (cell-, tissue- and gene-based treatments) is an unusual business for several reasons.
These are complex products. Exciting innovations are the result of deep understanding of the biology of the target disease states and are offered for translation to manufacture by expert biologist-inventors. It would be unrealistic to expect that such knowledge would necessarily be accompanied by a similarly deep understanding of regulatory affairs, process economics, operational research, manufacturing science, logistics and an understanding of clinical and patient pathways, and this is generally the case. Expertise in these topics is sought when new enterprise is set up. The unfortunate corollary of this situation is that the early proof-of-concept work and demonstration of efficacy will be based on a lab-scale process, which is unlikely to be directly scalable in a manner that conserves the desired characteristics. These will comprise the critical quality attributes, the target cost of goods and the criteria that make the process operable at large scale and suitable for incorporation into supply chains that may involve long-distance chilled or frozen transit. At the very least, extensive bridging studies will be indicated before Phase 3 clinical trials to establish comparability of early- to late-stage product.
While open innovation is becoming more frequent in the whole healthcare products sector, the advanced therapies arena operates to a larger extent than normal on the basis of innovations that arise from small, often academic, groups rather than big business [4,5].
The process engineer for advanced therapies is at a disadvantage compared with their historical counterparts in medicines manufacture based on chemistry or on fermentation-based processes. Chemical (and hence biochemical) engineering grew up around a series of bench-scale activities that were enlarged to plant scale using scaling criteria that were established along the journey. The result was, broadly speaking, that a manufacturing plant could be built around a set of large-scale pieces of equipment that were expected to provide service, and hence a spread in cost, over many different drugs with relatively minor variations in connectivity and sequence. The design criteria of the equipment would be known to the process engineers and, by the 1980s and 1990s, the empirical, step-wise approach to scale-up had been replaced in many companies by process simulation in silico, permitting large single-step increases in scale with safety and confidence and reduction in waste. A visitor to a pharmaceutical company from the post-war years up to the 1990s would expect to see a predictable series of manufacturing tools in the pilot and manufacturing plant (staffed by operators who understand the generic engineering principles of the kit, and treat it as product-agnostic). With the exception of stirred-tank fermenters and centrifuges, the same cannot be said of the equipment for manufacture of advanced therapies. Some platforms of manufacture using closed and semi-closed systems, such as Miltenyi Biotech’s CliniMACS Prodigy®, Octane’s CocoonTM and GE Healthcare Life Sciences’ XuriTM systems, are offered with the intention that they be used at full scale. Otherwise the pre-clinical innovation work is done largely at small scale using manual operations based around plastic disposables with the result that the impact on the product, of changes to process characteristics on scale-up is difficult to predict.
Process design is frequently based upon small-scale manual processes in the laboratory at the pre-clinical stage. It is therefore easier to scale-out than to scale-up as the latter will require additional investment of time and money in bridging studies if a different process is designed part-way through the development cycle.
Pursuing the synthetic drug analogy still further, we are now dealing with products that do not stand still. Unless the process scientists introduce a hold step, such as cryopreservation of cells or product, the manufacture and supply must take place on a timeline that is defined in the main by the natural behavior of the product.
This time-dependency is complicated further by the fact that, without the hold step, the product is changing during the transit stage and the administration. Some of the steps needed to administer the therapy may themselves be regarded as part of manufacture in the sense that the successful outcome depends on the willingness and ability of the healthcare professional to become an effective link in the chain of custody and to ensure that the way that they work in clinic is, in effect, the last step of GMP. Historically the distinction between manufacture and supply has been deemed to be so obvious that manufacture did not require a definition in the regulations. That is no longer the case and manufacture plus administration may be better thought of as a ‘service system’ [6].
The fixed costs of operation account for a high proportion of the total costs of manufacture. Anecdotally, the cost of quality (Quality Assurance plus Quality Control) can be as high as 30% of the total ex factory manufacturing cost. This is much higher than in, for example, the automotive industry.
There is an unusually strong relationship between know-how and the ability to operate processes reproducibly and with a high process capability. This reveals itself during late-stage development as a tendency to rely upon specific highly skilled manufacturing staff with experience that arises from the pre-clinical development phase of the product. Such reliance places the manufacturer at risk of interruptions to supply in the event of any later process changes that may be necessary, such as addition of a site to meet unusual demand or the departure of a key member of staff.
Little distinction is made between the intellectual property that relates uniquely to the product to be manufactured and that which is generic to the process that is used. This reveals itself as a reluctance to share know-how about process management that contrasts sharply, for example, with the commonplace sharing of knowledge about generic aspects of chemical processes that is normal among chemical engineers [7].
The transfer of technology from site to site is laborious and therefore undertaken only with reluctance. As the products can be challenging to transport and, in many cases, are best made close to the patient, this leads to limited patient access.
While the small numbers of manufactured units during the clinical trial stages of development may permit careful custody, by the time that full-scale manufacture is reached that state of affairs may be a luxury that can no longer be afforded. Fatigue, repetitive activities and sheer intensity of manual handling will conspire to increase the risk of mix-ups, contamination and batch loss post-launch. Small deficiencies in early process design will become major annoyances [8,9].
The marketplace clearly wants faster, more effective commercialization [3]. Recent regulatory initiatives such as the European Medicines Agency’s PRIME, the MHRA’s EAMS scheme [10] and the Breakthrough Therapy Designation in the USA are evidence of this. Patient expectations have been raised by the media and through easy access on the internet to information about trials [11,12]. Meanwhile, since 2008, it has become more difficult than usual to raise investment for business development that is likely to be ‘capital inefficient’, i.e. for which a large proportion of funds will go towards bespoke manufacturing installation, rather than utilizing existing frameworks as in the past [13,14]. The unusual unit operations of advanced therapies tend to make capital efficiency difficult unless the operation stays within the care of a CMO.
The preferred pathway to manufacture for launch
The characteristics of the sector mean that unless an innovator is able to sell the value proposition to a large existing company as their exit strategy they would be well advised to keep the operation under the shelter of an existing, probably academic, development facility with a GMP orientation as long as possible [14]. This works well with licensed facilities that have arisen in the university setting, for example the unit in the Orbsen building at NUI Galway, and those that operate to GMP-like conditions and can smoothly transfer the manufacture to a CMO, such as the Centre for Biological Engineering at Loughborough University and the Advanced Centre for Biological Engineering at University College London.
In the absence of adoption by big business the innovator faces a difficult choice: whether to attempt to establish their own facility with appropriate compliance or to stay long-term within the walls of a CMO such as the Tigenix-Cognate Bioservices arrangement at Memphis, Tennessee.
While research has been going on in cell- and tissue-based therapies for several decades and commercialization since the 1990s, there has been little operational research. In fact, research into business models began at about the same time as the first companies, such as Advanced Tissue Sciences, began supplying [15]. In short, there has been, and is, a lot of investment in advanced therapy R&D but relatively little in how to manufacture in a way that provides the features listed above. Perhaps logistics can be turned into a strategic tool for growth [6]. We have been here before in another sector.
Alternative Enterprise Formats
In the late 1990s and the early 2000s the concept of the Collaborative Enterprise was examined and promoted by some of the Regional Development Agencies in the UK [16]. The interest had been sparked by experience overseas, particularly in the Swiss microelectronics industry [17], where a sector downturn had occurred and companies needed to find ways of remaining operational and flourishing while incurring lower fixed costs of operation.
In order to stimulate business development, generate more regional jobs and create better access to the resources necessary for operating high-technology SMEs, two models of operation were put forward in particular. These have some similarities and one is a development of the other.
In July 2016, these two ideas were examined in a one-day informal workshop at Kegworth chaired by Loughborough University and funded from the Health and Wellbeing at Loughborough (‘HWB@LU’) budget (“Collaborative Enterprise Networks for Aseptic Products, a Model for the Midlands?”) [18]. The purpose of the workshop was to bring together key opinion-leaders from the NHS Trusts in the region (East and West Midlands) with representation from the regional Academic Health Science Networks, to examine the strengths and weaknesses of an approach that has the capacity, at least in principle, to satisfy these requirements. This approach is referred to as a Collaborative Enterprise [19] and is a specific form of the operation more generally known as an Extended Enterprise.
Extended Enterprises
The Extended Enterprise (EE) has its origins in the management of supply chains [20] and has been applied commercially in several sectors [21–23]. Its character is fundamentally relational rather than purely customer–supplier. The relationships fall broadly into two categories: those outside the EE, where the familiar transactional relationships apply in which vendor and customer act to maximize their personal advantage through negotiation, and those within the EE where the relationships are collaborative rather than adversarial because of their long-term persistence and the mutual advantage.
The primary motives for creating an EE can be listed as:
- Cost reduction through the avoidance of vertical integration of the necessary services within each collaborating organization. Resources are instead shared throughout the network;
- Management of fluctuations in demand through spreading the operational capacity over more than one center;
- Recruitment of customers through multiple entry points throughout the enterprise, leading to distribution of opportunities to mutual benefit that would otherwise be restricted to one party or merely declined due to lack of capacity or poor ‘fit’;
- The ability of each actor to focus on their own area of excellence rather than attempting to address all the aptitudes that are needed to manufacture, supply and react to market changes;
Stability in time by creating an enterprise that is resilient to market forces through enhanced horizon-scanning, greater expertise and higher purchasing power than would be possible if the actors were separate; - Greater market reach and penetration through regional nodes that can supply markets with differing requirements or in different locations;
- Optionally, an increase in the pace of innovation through an open innovation model.
Collaborative Enterprises
A Collaborative Enterprise (CE) or Collaborative Enterprise Network (CEN) has the advantages of the EE and acts as a single business when appropriate, permitting the partner organizations to operate independently as well [17]. It is “a … business entity of legally independent, … selected enterprises, which, due to a common purpose coordinate the operation of sub-tasks through negotiation and agreement [24]”.
Shared ownership of outcomes is managed through operation via an over-arching legal entity with representation at Board level from the collaborating organizations.
A review of the literature [24] produced a set of five features that are generally characteristic of any CEN. They are:
- The presence of at least one collaborative purpose
- The presence of at least one collaborative task
- The necessity of selecting members
- The organized structure and system of rules of collaborations
- The existence of legal regulations of the CEN concerning the internal and external representation
There are similarities to the Extended Enterprise [20]:
- Both emphasize a close relationship upstream and downstream (suppliers and customers);
- Both emphasize containment of development costs and reduction in innovation time;
- Both employ an open innovation model, drawing on partners’ skills as necessary to supply products from outside innovators and inventions from within;
- The collaborators participate early in planned innovation based upon explicit identification of their collective and individual capabilities, know-how and limitations;
- The EE and the CE aim to prosper by applying knowledge and resources to the mutual benefit of the collaborators;
- The two key ‘enablers’ for the enterprise are technology (without which the CE could not remain agile) and trust (without which the collaborators would break away to their own competitive advantage).
The role of customers is vital to the CE. In the context of a CE for advanced therapies, this key relationship can be viewed as a two-way flow of information with products flowing from manufacturer to customer. On the one hand the clinics provide insights and forecasts that allow the production features of the CE to adapt and optimize their supply model. On the other, the close communication between user and manufacturer has the capacity to create a just-in-time variant, for advanced therapies, of the more familiar ‘Specials’ supplier [25–28].
Even under existing business arrangements there is a much greater degree of shared responsibility between healthcare professional and manufacturer for the safety and efficacy of an advanced therapy medicinal product (ATMP) treatment upon administration than there is for a more traditional medicinal product. Rather than the hand-off transaction of purchase and transfer of ownership the role of the healthcare professional effectively supplies the final stages of manufacture. This is particularly clear when considering the measures necessary to dress and apply a tissue implant. Under these circumstances the role of customer is shared between the patient (who wishes an effective treatment), the healthcare provider (who has requirements that must be met in order to execute their part in the chain of custody) and the clinic itself (who will have an interest in the balance of cost of the treatment and the cost of the facilities in which the last hundred meters of custody takes place [29]. The enterprise will need to make a decision about which manufacturing platform technologies to adopt (production, quality control, communications, track-and-trace) to satisfy these stakeholders and there will need to be some careful analysis of the core business intention in order to target capital investment at the needs of the true customer at each point.
Differences from Trading Associations
It can be argued that the CEN concept is simply an extension of the familiar idea of a trading association. Trading associations have been long established and have operated with varying degrees of success. The main weakness of a trade association is the tendency of the individual parties to break away and seek commercial advantage through independent activity in direct competition with the remaining members of the TA. This disadvantage arises from the main purpose of the TA, which is normally to act as a form of protective practice. In the case of a CE, the mutual advantage arises from the shared resources and from access to manufacturing and supply deals that may not be operable by individual parties acting alone.
What Characteristics will a Collaborative Enterprise for Advanced Therapies possess?
To derive a typology of CEs is difficult as there has been no generally accepted morphological model [24]. Instead it is more helpful to describe the features of a CE that would be advantageous to a community of interest that intends to manufacture and supply advanced therapies.
What we are talking about is a geographically based cluster for the aseptic healthcare products sector [30]. Within this the manufacturing research group is a single node in the network of relationships.
What form should the collaboration take between the participants?
The typology of useful CEs for ATMPs can then be defined by proposing and examining the main features that such a system of systems needs to possess in order to be of value. This can be generated from a stakeholder analysis as shown in (Table 1). Note that more than one collaborator in each category may be present in a CEN and that for any one node the manufacturing site may be in a long-term collaboration with preferred process development collaborators, who could be academic.
| Table 1: Stakeholder table (needs analysis). | |||
|---|---|---|---|
| Participant | Direct needs | Indirect needs (all relate to business sustainability) | Advantageous role |
| Innovator | Receipt of a revenue stream or payment for a direct buy-out | Esteem; credibility to pursue further innovation; protection of ‘back of shop IP’ | Member of an Innovation Pool nurtured over the long term, e.g. offering technology options to the CE Board |
| Development team | Revenue stream | Deep and growing expertise in specified unit operations | Preferred supplier of services |
| Manufacturer | Revenue stream | Recognition as the ‘go-to’ centre for a chosen technology platforms; availability of tools and techniques from more than one supplier | Preferred supplier of services |
| Carrier | Revenue stream; non-fragmentary volume of work (scale) | Establishment as the carrier of choice due to: a) reliability, b) flexible carrier modes, c) economies of scale | Preferred supplier of services |
| Regulator | Monitoring of compliance; Accelerated access to safe, cost-effective treatments | Establishment of preferred tools and techniques from more than one supplier | Promotion of best practice through scientific advisory route |
| Equipment factors | Reliable revenue stream based on acceptance of equipment as ‘default’ | User requirement specifications that enable confident new product development | Provider of goods and services; partner in process development through equipment evaluation |
| Users (healthcare professionals) | Ease of access to safe, effective treatments | Supply arrangements that fit with clinical and procurement practices | Partner in development |
| Patients | Effective treatment | Ease of access, early access; Patient-focused treatment pathway | Informed early recipients of products |
| Investors | Return on investment | Acceptably low risks; avoidance of capital-intensive proposals | Preferred source of investment as options |
| Regional society | Job creation, improved healthcare | Ease of patient access | Host market |
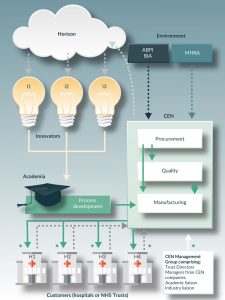
Figure 1. Format of a CEN derived by the Kegworth workshop. Solid arrows denote flow of materials/products and information. Dotted arrows denote flow of information. Asterisks denote a relationship of shared resources.
An illustration of an archetypal CE for the UK context will help and the description here is the one that was discussed and approved at the workshop described above. The system is shown diagrammatically in (Figure 1). It comprises a non-adversarial business system co-owned by a group of organizations to include investors, NHS Trust directors, clinicians and license holders for the GMP manufacturing site and operated for mutual benefit by making shared use of some of the resources of the Trusts. The shared resources would be, for example, negotiating leverage for purchasing of raw materials, use of existing logistics and manufacturing system and access to NHS employees (nurses, clinicians) and departments (IT, Finance, Procurement). The system would undertake clinical, managerial and research activities in order to accelerate the delivery of conventional aseptic products, where appropriate, and new advanced therapies. The CE would operate to ‘just-in-time’ principles and would provide a delivery platform enabling the easy transfer of operational knowledge into process development. The CE would be based on a shared belief that an economical and beneficial service can be provided whilst recognizing the constraints coming from patient perceptions, market conditions, availability of necessary structures with the healthcare Trusts and the regulations.
The current regulatory oversight for medicines in the UK is subject to the Human Medicines Regulations 2012 (SI 2012/1916), which include the transposition into regional law of Article 5(1) of EU Directive 2001/83/EC on medicinal products [26]. The Directive and the UK Regulations allow for exemptions from these provisions under certain circumstances. One category of exemption route may lead to market access at an earlier stage in product development than the usual Phase 3 clinical trials followed by application for a Marketing Authorisation (MA). This ‘Specials’ category [25,28], if used responsibly and within the intention of the regulation, permits the supply of limited quantities of a product on a non-routine basis without the prior acquisition of a MA. Such a product must be supplied only in response to an unsolicited order and must be used only in cases of ‘special need’, hence the name, in cases where there is no fully licensed equivalent product. ‘Specials’ may not be supplied for use outside the European Economic Area. This arrangement implies close communication between clinician and manufacturer and agreement with the patient or their representative. The ‘Specials’ route is not a substitute for the clinical evidence that is needed for a MA Application. Neither does it exempt the manufacturer from the obligations of Good Manufacturing Practice. The ‘Specials’ route may not readily be used to glean clinical data from a hospital because that would make the patient an unwitting participant in a clinical trial. Nevertheless, the application of a product as a ‘Special’ carries with it the obligation to collect and report adverse reaction information and knowledge that there has been effective, though limited, use of a product as a ‘Special’ could be a contributing factor in increasing the confidence needed to support investment in full-scale manufacturing development at a later stage.
Is there a place for the Franchised Operation?
When manufacturing start-ups begin in the advanced therapies sector, in order to comply with GMP and GDP it is first necessary for them to create a Quality Management System (QMS) of sufficient scope and detail to address the important business processes under a Site Master File and accompanied by a library of SOPs and Controlled Documents to define practice. The key elements of training (together with proof of competency), procurement processes, supplier audit schedules, responsive (on-call?) support engineering, specialist microbiology services and accurate track-and-trace (perhaps with predictive ordering) are all essential elements for such a small operation and contribute to the fixed, indirect overhead costs of the operation when it is running, and to much management time during set-up. Add to this the necessity to arrange suitable supply agreements with the clinics and an intimidating hurdle in time and money appears in the business plan.
Recently the sector has seen the emergence of joint ventures and resource-sharing arrangements between companies. Examples are the Cognate-Tigenix agreement, the Lonza-Sanofi JV and the Smith & Nephew-ATS JV. The drivers behind these agreements include the advantages of resource-sharing and rapid set-up.
Taken to its logical conclusion, these needs and drivers may be satisfied by that well-established business format, the franchise.
The advantages of operating as a franchise can be listed as (for the franchise taker):
- Boilerplate QMS
- Established (re)training packages
- Agreement templates (quality, supply)
A degree of support service to be matched to the portfolio needs - Buying into an established reputation and management processes
- Lower start-up costs than establishing the operation from scratch
- Validation and qualification packages (that still need to be executed)
- Support services for equipment failure
- Negotiating power with upstream suppliers as a result of membership of a larger operation
- Standardization of techniques
- Access to expertise
- Higher probability of success than going it alone
…and for the franchise giver:
- Distribution of manufacture (thus avoiding the need to generate revenue from single factories that may be difficult to operate at scale for out-scaled and/or autologous processes)
- Access to market regions via a hub-and-spoke network
- Progressive growth of a revenue stream
- Economies of scale in providing the necessary support to several franchise-holders
- Repeat business from the franchise holders
- Income from an agile, responsive network
- Market development by the franchisee
The concept of a franchised operation carries some risks. These arise in the specific context of the regulatory constraints that apply to all medicinal products and to ATMPs in particular.
For a centralized, single-owner manufacturing company there is no ambiguity in the current regulations about the process by which a medicinal product is released onto the market or to a patient. In the EU, it is obligatory for that decision to be made by a Qualified Person (QP). The QP bases their decision on two pieces of information. The compliance of the batch to specification is necessary but insufficient condition to enable release and it is supported in practice by the QP’s knowledge of, and confidence in, the competent and GMP-compliant operation of the manufacturing facility. This last point can only be assured through a program of audits, site visits, training, preventative maintenance, corrective and preventative action and Quality Reviews with the management. In the case of a de-centralized enterprise, franchised or single-owner, the same degree of assurance of quality must be maintained if patient safety is to be protected. If a franchise-taker is negligent and damages the reputation of the franchise through malpractice or non-compliance then the effect can negatively impact the whole network. To mitigate this risk the concept of licensure of the franchisee may be based on quality oversight from the Hub. Such oversight is desirable at several levels: as a support service (locum QP, QA management), detection of and response to manufacturing trends and dealing early with resource shortfalls or poor use of resources. The complexity and depth of such oversight will need to be much higher than is normal for conventional franchises. A system of oversight is needed which confers on the QP, acting on behalf of the franchise-giver, the authority, the resources and the capacity to discharge their duties at an equivalent level to the current centralized model. This feature, without which the de-centralized model cannot be operated effectively, will need to be addressed with appropriate operational research in which the issues of data management and secure transmission, rotas of inspection, automation (with monitoring at a distance) and the introduction of intermittent testing of the staff and the equipment to verify that manufacture is capable of delivering goods of adequate quality and of screening out defective product.
Who holds the MA in a franchised business? A feature of the arrangement is that the franchise taker is gaining expertise so the giver is best placed to manage the process for this yet the locations of the Spoke and the Hub will be different. Again we are back to a CE model in which a central business entity holds overall responsibility and enforces compliance through responsible management of the franchise license, which is dependent upon the holder’s management of the MA for their site. These points are, to the best knowledge of the author, yet to be debated with a regulatory authority and would form a useful topic for an industry-regulatory workshop. They are of dominant importance for the viability of de-centralized manufacture and will require an extension of the current reporting arrangements for single-site operations in which the quality professionals report separately from production staff to senior management to avoid conflicts of interest. In the de-centralized case, a peripatetic arm of the quality unit, accountable only to the franchise giver, may be the only viable solution.
How can the franchise offer be so structured that the multiplicity of products can be managed economically? The most likely route to manage this is by reducing the options for manufacturing process design to a small set of types, around which a given site can vary the steps to accommodate several products. This typology of manufacture for advanced therapies needs to be available for the community in a practical, standardized form if it is to be useful in the future and there are a few ways of making the knowledge explicit. The use of ‘partial models’ of processes set in the context of overall operations can be achieved by applying standard IDEF0, an internationally-recognized form of the operational modeling technique known as Structured Activity and Design Technique [31–34]. This approach allows the creation of reconfigurable, customizable models in a similar way that, say, computer programs are built from reusable blocks of code and tailored to the application [35]. Inclusion of cost roll-ups in the models, a feature of some commercial SADT modeling software, would then allow rapid examination of the total costs of operation to different structures, enabling choices to be made between them based on present savings and future strategy for growth. At present such calculations are rarely addressed in planning for delivery of advanced therapies and then only with difficulty. The SADT approach allows the total costs of the operation to be estimated, including management, transport and support services, in a way that allows apportionment of overhead to different products if that is relevant to strategic decision-making [36].
Signposts to the future
The importance of innovation in operational and business models when commercializing cell-based therapies is shown in some recent creative examples of real-world product translation. Novartis enjoys an advanced position in the commercialization of their CAR-T cell therapy Kymriah™ and has encountered two of the issues described above. Their work to date has capitalized on a productive relationship with an existing center, in this case the University of Pennsylvania. Novartis has also carefully considered the implications of centralized versus de-centralized operation for their manufacturing process, which involves two interactions with the patient, one involving harvest of cells (followed by transfer to manufacturing facility, genetic manipulation and expansion of the cells) and one involving administration of the expanded population to the patient. The option to move to regional manufacture-and-treatment centers offers the potential to reduce the risks involved in the two-way supply chain and to facilitate clinical coordination [37]. The Morris Plains facility appears to offer the potential to act as a training and refresher center for manufacturing staff on other sites. It could therefore act as a ‘franchise prototype’, a manufacturing and training center from which training and assessment can take place that will enable satellite operations. Novartis is currently working on a centralized model and this will satisfy the modest numbers of units that must be manufactured each year. The Company rationalized its R&D base in 2016 [38]. If the decision is taken to extend the platform technology to other indications that require larger manufacturing volumes then the de-centralized model will increase in its relevance to Novartis’ commercial growth.
The Milan-based Fondazione Telethon San Raffaele is an example of the creative use of business structure and financing in order to facilitate the investment in, and execution of, cell therapy product development. This not-for-profit organization was founded in 1990 to provide treatments for rare diseases and combines rigorous screening of candidate projects, using an independent scientific and medical committee, with close management of partner institutes avoiding the use of liaison organizations. The Foundation has formed an operation based around a management board that coordinates funding granted to hospitals, universities and other research technology institutes associated with the R&D program. The model is certainly productive with four-figure numbers of researchers now involved and in excess of 2000 projects funded to date. Nearly 500 diseases have so far been studied and nearly half a billion euros raised in investments [39].
Is there an Alternative?
In order for the sector to flourish, it would be helpful for many categories of advanced therapy manufacturers to establish operations with the following characteristics:
- Reduction of the fixed costs of operation, so far as is reasonably practical, by sharing existing resources with the clinics to which they supply. Many hospitals already have just-in-time procurement and order fulfillment systems for medicines or transplantable goods that are of short shelf life and/or which have temperature and shock sensitivity;
- Early access to the patients, subject to responsible controls within the current legislation, in order to generate early revenue for the innovators, thus creating experience that, while it will not replace the need for clinical trials, will generate greater investor confidence in financing the trials when the time comes [40];
- A small set of well-established pathways for patient recruitment, subject to prudent and responsible qualification criteria based on the usual risk-to-benefit assessments, agreed with the regulatory authority and NHS England;
- An in-built personal motive for each actor in the chain of custody to manage the quality of the product proactively;
- A network of centers that promote and share best practice in manufacture using characteristic unit operations and who distinguish between, so to speak, IP at the ‘front of the shop’, i.e. generic capability based on a deep and growing understanding of the critical control points in the unit operations in which they excel and IP at ‘the back of the shop’, i.e. know-how that is unique to the products that they manufacture.
</span>
The concept of the Collaborative Enterprise has the potential to satisfy several, if not all, of these criteria and is a worthy topic for future research and evaluation.
Conclusion
In conclusion, advanced therapies present many technical challenges in their development and these can distract from the importance of applied research into operational choices for viable, sustainable chains of manufacture and supply. Precedent for supply chains based upon novel relationships between inventor, CDMO, manufacturer and customer exist already (and there will be more) in the business and operational research literature for different sectors with characteristics that are shared with advanced therapies. No single precedent is likely to fit without modification, careful testing and scrutiny by the regulatory authority, but the rewards may be significant for those that are willing to investigate the possibilities. It is time for the systems engineers to meet up with the quality professionals and examine the potential.
Financial & competing interests disclosure
The author is a Visiting Professor of Regenerative Medicine Manufacture at Loughborough University and has no relevant financial interests in any of the entities involved in the paper. No writing assistance was utilized in the production of this manuscript.
ACKNOWLEDGEMENTS
The author would like to acknowledge the support of Dr Antuela Tako and Dr Nicola Bateman (both at the time were Senior Lecturers in the School of Business and Economics at Loughborough University) in the organization and delivery of the Kegworth Workshop at which the format of Figure 1 was derived.
References
1. Aldridge S. Facing up to the cell therapy challenge. Pharmaceutical Technology Europe. Advanstar Communications Inc. 2007; 19 (4): 14–7.
2. Buchanan DA. The limitations and opportunities of business process re-engineering in a politicized organizational climate. Human Relations 1997; 50(1): 51–72.
CrossRef
3. Gardner J, Webster A. The social management of biomedical novelty: Facilitating translation in regenerative medicine. Soc. Sci. Med. 2016; 156: 90–7.
CrossRef
4. Salmikangas P, Celis P. Current challenges in the development of cell-based medicinal products. Regulatory Rapporteur 2011; 8(7–8): 4–7.
5. Hildebrandt M, Sethe S. Caught in the gap: ATMP manufacture in academia. ISCT Telegraft 2012; 19(1): Website
6. Nordström KM, Närhi MO, Vepsäläinen APJ. Services for distribution of tissue engineering products and therapies. Int. J. Prod. Perf. Manag. 2009; 58(1): 11–28.
CrossRef
7. Mansnérus J. Commercialisation of Advanced Therapies A Study of the EU Regulation on Advanced Therapy Medical Products. 2016. Academic Dissertation. Faculty of Law, University of Helsinki: Website
8. Warren RS. (Senior Director, RA/QA, Smith & Nephew Wound Management, La Jolla.) Personal interview with the author, 2006.
9. Mason C. The time has come to engineer tissues and not just tissue engineer. Reg. Med. 2006; 1(3): 303–6.
CrossRef
10. Medicines and Healthcare Products Regulatory Agency. Guidance: Apply for the early access to medicines scheme. 2014: Website
11. Lee D. Patient expectations: A surgeon’s perspective. MPS Casebook 2015; 23(1): 8–9.
12. Gremeaux V, Coudeyre E. The Internet and the therapeutic education of patients: A systematic review of the literature. Ann. Phys. Rehab. Med. 2010; 53: 669–92.
CrossRef
13. Bonfiglio GA. Investor Forum, Horizon Scoping: Why invest in RM? In: World Stem Cell and Regenerative Medicine Congress Investor Forum, London, May 22. 2015: Website
14. Omidvar O, De Grijs M, Castle D, Mittra J, Rosiello A, Tait J. Regenerative medicine: Business models, venture capital and the funding gap. Innogen and Economic and Social Research Council report 2014: Website
15. Zott C, Amit R, Massa L. The business model: Recent developments and future research. J. Manage. 2011; 37(4): 1019–42. CrossRef
16. Mawson J. Social enterprise, strategic networks and regional development. Int. J. Sociol. Soc. Pol. 2010; 30(1/2): 66–83.
CrossRef
17. Cheikhrouhou N, Pouly M, Huber C, Beeler J. Lessons learned from the lifecycle management of collaborative enterprises networks. J. Manufact. Tech. Manag. 2012; 23(8): 1129–50.
CrossRef
18. Phillips W, Medcalf N, Dalgarno K et al. Re-distributed manufacturing in healthcare; Creating new value through disruptive innovation: Website
19. Halal WE. The collaborative enterprise: A stakeholder model uniting profitability and responsibility. J. Corp. Citizen. 2001; 2: 27–42.
CrossRef
20. Filieri R, Alguezaui S. Extending the enterprise for improved innovation. J. Bus. Strat. 2012; 33(3): 40–7.
CrossRef
21. Cullen P-A. Contracting, co-operative relations and extended enterprises. Technovation 2000; 20(7): 363–72.
CrossRef
22. Lin G, Ettl M et al. Extended-enterprise supply-chain management at IBM Personal Systems Group and other divisions. Interfaces 2000; 30(1): 7–25.
CrossRef
23. Davis B. Managing the extended enterprise. Prof. Eng. 2003; 16(11): 39–40.
24. Baum H, Schütze J. A model of collaborative enterprise networks (45th CIRP Conference on Manufacturing Systems 2012). Procedia CIRP 3. 2012; 549–54.
25. Faulkner A. Special treatment? Exceptions and exemptions in the politics of regenerative medicine gatekeeping in the UK in global context. ESRC Rising Powers Research Working Paper No. 46. 2015:Website
26. European Parliament and Council. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code relating to medicinal products for human use (Article 5 (1)). Official Journal L – 311, 28/11/2004, 67–128.
27. Medicines and Healthcare products Regulatory Agency. Regulation (EC) No. 1394/2007 on Advanced Therapy Medicinal Products (“The ATMP Regulation”): Guidance on the UK’s Arrangements under the Hospital Exemption Scheme: Website
28. Medicinal and Healthcare products Regulatory Agency. The supply of unlicensed medicinal products (“specials”). MHRA Guidance Note 14. 2014: Website
29. Malik NN, Durdy M. Commercialisation of CAR T-cell therapies: Business model spectrum. Drug Disc. Today 2016; 22(1): 1–4.
CrossRef
30. Taticchi P, Cagnazzo L, Beach R, Barber K. A management framework for organisational networks: a case study. J. Manufact. Tech. Manage. 2012; 23(5): 593–614.
CrossRef
31. Cheng-Leong A. Enactment of IDEF0 models. Int. J. Prod. Res. 1999; 37: 3383–97.
CrossRef
32. Cheng-Leong A, Pheng KL, Leng GRK. IDEF*: A comprehensive modelling methodology for the development of manufacturing enterprise systems. Int. J. Prod. Res. 1999; 37: 3839–58.
CrossRef
33. National Institute of Standards and Technology. Draft Federal Information Processing Standards Publication 183. Announcing the standard for Integration Definition for Function Modeling IDEF0. Gaithersburg, National Technical Information Service. 1993.
34. Mayer RJ, Painter MK, Dewitte PS. IDEF family of methods for concurrent engineering and business re-engineering applications. Knowledge Based Systems, Inc. 1992: Website
35. Cantamessa, M, Paolucci E. Using organizational analysis and IDEF0 for enterprise modelling in SMEs. Int. J. Comp. Int. Manufact. 1998; 11: 416–29.
CrossRef
36. Dowless RM. Using activity-based costing to guide strategic decision making. Health. Fin. Manag. 1997; 51(6): 86, 88, 90.
37. Yin P. 3 Keys to scale up CAR T-cell therapy manufacturing. Bioprocess Online. 2017: Website
38. Palmer E. Novartis commits to CAR-T manufacturing in restructure of cell therapy work. FiercePharma. 2016: https://www.fiercepharma.com/manufacturing/novartis-commits-to-car-t-manufacturing-restructure-cell-therapy-work
39. Telethon. 2018. Our visiting card: Website
40. Accelerated Access Review: Final report. 2016: Website
Affiliation
Nicholas Medcalf
Innovation Lead, Advanced Therapies, Innovate UK, Polaris House, Swindon, North Star Avenue, Swindon, SN2 1FL, UK.
+44 07584 154539
nicholas.medcalf@innovateuk.gov.uk
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</