Addressing scaling-up limitations: optimized PEI-mediated production of clinical grade viral vectors
Cell Gene Therapy Insights 2018; 4(2), 71-79.
10.18609/cgti.2018.013
With the progress in developing new viral vector systems guided by safety, specificity and potency considerations, several gene and cell-based therapies are now close to being clinically approved and commercially available to treat genetic diseases. These viral vectors systems are based on the production of mainly adeno-associated viruses (AAV) and lentiviruses by transient transfection of human embryonic kidney (HEK)-293 derived producer cell lines. Virus vector production using the right transient transfection method is crucial to provide the flexibility and reproducibility that is needed to scale-up from initial process development to manufacturing of high-quality grade viral vectors. PEI-mediated transient transfection is an efficient and cost-effective approach to produce viral vectors. With the available quality grades of PEI, PEIpro® and PEIpro-HQ®, Polyplus-transfection offers a reliable PEI transfection method suitable for process development up to viral vector production for clinical and commercial manufacturing.
Initially developed for the treatment of hereditary single-gene defects, gene and cell-based therapies have grown in number to target acquired diseases. The growing number of ongoing gene therapy trials is a clear indication of the progress and flourishing success of virus-based approaches for diseases with no current viable treatment options. The past year has marked an important milestone in the number of Food and Drug Administration (FDA) approved cell and gene therapies, with chimeric antigen receptors (CAR)-T cell therapies Kymriah™ and Yescarta™, and the gene therapy Luxturna™. Kymriah™ and Yescarta™ CAR-T are immunocellular therapies developed to respectively target B-cell precursor acute lymphoblastic leukemia and large B-cell lymphoma, whereas Luxturna™ is a AAV2-based gene therapy to treat a form of hereditary blindness [1,2]. Recrudescence of viral vector-based gene and cell therapy preclinical and clinical trials is attributed to the advances made in developing new viral vector systems guided by safety, specificity and potency considerations, as well as advances made in equipment and process methodology.
One of the current bottlenecks that needs to be addressed is the transition from small-scale process development towards large-scale manufacturing to support advanced clinical trials and in fine commercialization needs. Upscaling of virus production is a challenging multi-faceted process, and therefore any simplification is welcomed. Transient transfection remains the preferred method for virus production. It is a straightforward delivery method that brings the needed flexibility to accelerate process development, and facilitate transfer of process from small- to large-scale production [3].
Advantages of transient transfection for large-scale virus manufacturing
Considerable efforts have been made to facilitate transient transfection, in terms of cell lines, transfection reagent and cell culture-based systems, to ensure high viral titer yields during scale-up for preclinical and clinical applications. Transient transfection is a simple approach that allows delivery of genetic material and ensures immediate production of viral particles with no genome integration. Several transient transfection methods have been used to prepare small- up to mid-scale virus production, including calcium phosphate, cationic lipids and polyethylenimine (PEI).
Calcium phosphate is often regarded as the most economical option in research laboratories. While this may be true in terms of cost of raw material, it remains a cumbersome and variable transfection method that requires rigorous control of several environmental parameters (pH, calcium and phosphate concentrations), large amounts of plasmid DNA, as well as the presence of serum to circumvent toxicity. The use of cationic lipids leads to less inter-experimental variability, the imputed cost and cellular toxicity are detrimental to their use in large-scale virus vector production [4].
PEI is widely used due to its affordability and high DNA delivery efficiency, in both adherent and suspension cells grown in serum-free medium [5,6]. Several PEIs of different molecular weights and chemical properties that are not specifically developed for transfection are commercially available. Based on the available quality controls, these PEIs are poorly characterized in terms of chemical structure and purity, and therefore cannot guarantee batch-to-batch reproducibility in transfection efficiency. With the extensive characterization and Quality Controls defined by current Good Manufacturing Practice (cGMP) guidelines for large scale virus production, the transfection reagent PEI must be sourced from qualified suppliers, and have gone through rigorous testing to ensure reproducible transfection efficiencies and virus titer yields.
PEIpro® for large-scale virus production
PEIpro® (Polyplus-transfection, France) is suited for small- to large-scale production of various viruses, notably lentiviruses, AAV and virus-like particles [7–9]. PEIpro® is a rigorously qualified PEI that benefits from extensive research development in polymer chemistry and formulation: polymer structure, deacetylation degree, polydispersity index and molecular weight. The polymer branching structure is directly correlated with the availability of protonable amines, which affects the global charge of the polymer. Formation of positively charged reagent/DNA complexes is essential to obtain a higher transfection efficiency, rendering linear PEIs preferable to branched PEI [10,11]. The polydispersity index of PEI, which corresponds to the distribution of its molecular mass should be close to 1, meaning that the final PEI is composed of only one length of PEI (Figure 1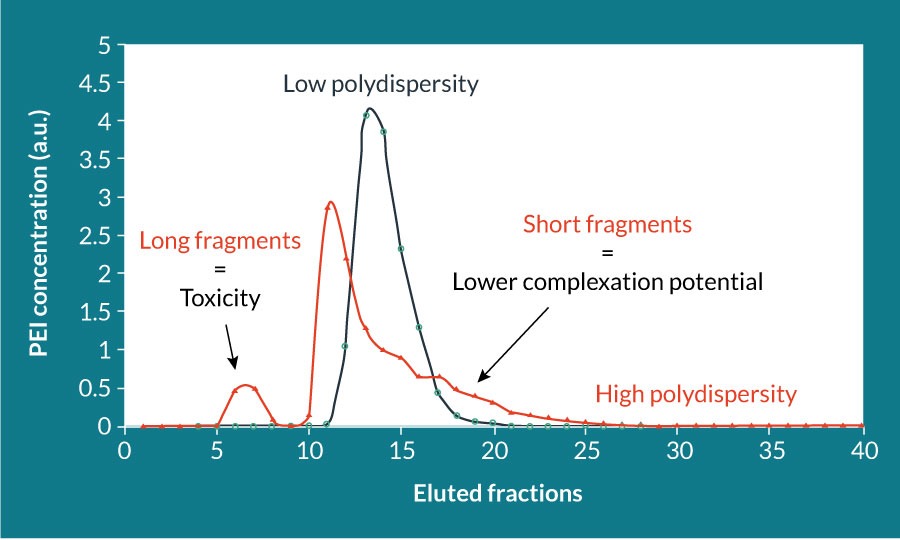
Remaining challenges
Choosing a cell culture system
During process development for viral production, optimization of virus production is often achieved in adherently grown cells, as this remains the standard traditional laboratory set-up. Depending on the type of viral vector, different subtypes of HEK-293 cells are preferred such as HEK-293 for AAV virus production, and HEK-293T for AAV and lentivirus production [3]. Commonly used adherent-based cell systems (flasks, cell factories and roller bottles) are generally difficult to scale-up, as they favor mishandling risks during the increased processing time that manipulation of a higher number of culture vessels requires. A variety of alternative culture vessels have thus been developed, while acknowledging time and space concerns.
Fixed-bed culture systems such as iCELLis® bioreactors (Pall Corporation, USA) are compatible with the use of PEIpro®. The iCELLis® is an innovative solution to increase volumetric productivity. Use of this high-cell density system in combination with the use of PEIpro® has led to substantial increase in viral titer yields, reaching AAV viral titer yields of 9.0 x 1014 virus particles per square meter of fixed bed of the iCELLis® Nano [6]. Recently, an elegant study by Valkama et al., 2017 has demonstrated the reliability and scalability of clinical scale lentivirus production in the iCELLis® 500 using PEIpro® [13].
Switching to suspension serum-free cell culture-based systems can offer considerable advantages in simplifying the scaling-up process, in terms of cell culture conditions and downstream viral vector purification steps. More and more virus producers are moving towards animal-free components, and for this several animal-free synthetic media are commercially available to maintain high productivity [4]. PEIpro® offers the flexibility that is needed during the upscaling process, as it is compatible with most of the available mammalian cell-based transient trasfection systems for virus production. Regardless of cell type, culture conditions, culture vessels and production scale, this optimized PEI-based transfection reagent has led to high AAV and lentivirus vector yields (Table 1).
| Table 1: PEIpro® reagent supports virus production runs in most cell culture systems in both adherent and suspension cells. | |||
|---|---|---|---|
| Cell culture system | Vector | Cells | Titer |
| CS10®/CF10® | AAV | Adherent HEK-293, HEK-293T | 1011-1013 VG/mL |
| Fixed-bed bioreactor (iCELLIS®) | AAV | Adherent HEK-293T | 1014-1016 VG total |
| Shaker Flask | AAV | Suspension HEK-293, HEK-293T | 109-1010 VP/ml |
| Bioreactor (stirred tank) | AAV | Suspension HEK-293, HEK-293T | 0.8-1.5 x109-1010 VG/mL |
| 10 cm dish/75 cm2 | Lentivirus | Adherent HEK-293, HEK-293T | 1-2 x108 TU/mL |
| HYPERflask®/HYPERstack® | Lentivirus | Adherent HEK-293, HEK-293T | 1-2 x108 TU/mL |
| Shaker Flask | Lentivirus | Suspension HEK-293F, HEK-293T | 2x 107-1010 VP/mL |
| Bioreactor (stirred tank) | Lentivirus | Suspension HEK-293, HEK-293T | 107 IG/mL |
| Irrespective of the cell culture system and production scale, PEIpro® and PEIpro®-HQ have led to high AAV and lentivirus vector yields. This table aims to provide data on titer yields achieved, and is not intended for direct comparison. Data is provided by collaborators and is previously unpublished. Titer yields are measured as physical titers in the form of viral particle (VP) and viral genome (VG), or as infectious titers in the form of transduction unit (TU) and infectious genome (IG) per ml of cell culture. | |||
Improving process performance
In addition to choosing the optimal cell culture system, it is important to maintain process performance, as well as comply with quality requirements for clinical-grade virus production. Maintaining comparable virus titer yields and infectivity during the scale-up process using PEI-based transfection approach is achievable with PEIpro®. Transient transfection at a large scale implies preparing larger volumes of DNA and transfection reagent mix before adding the complexes to the cell culture. It can therefore be challenging to maintain reliable transfection efficiency when scaling up, and to this end it is important to identify the minimal volume needed to prepare complexes in, as well the time frame encompassing efficient complexation and addition to cells.
Answering quality requirements
With the great momentum in viral vector-based gene therapy, the need for cGMP manufacturing has become a major concern, in terms of large-scale production and quality requirements for future commercialization. With the extensive characterization and Quality Controls that are required for cGMP viral vector production, PEI must be sourced from qualified suppliers, and have gone through rigorous testing to ensure reliable transfection efficiencies and hence reproducible virus production. To fulfill all the quality requirements associated with the use of PEIpro® in manufacturing of recombinant viral vectors for human clinical trials, Polyplus-transfection® supplies a higher quality grade PEIpro®, PEIpro®-HQ. PEIpro® and PEIpro®-HQ are fully synthetic animal-free reagents identical in chemistry and formulation. Therefore, protocols established with PEIpro® during the initial process development stage can be used with PEIpro®-HQ to keep producing identical virus titer yields during clinical batch manufacturing.
PEIpro®-HQ is produced and formulated following a strict manufacturing process, with, in addition, extensive characterization and quality testing to ensure that each produced batch fulfills the pre-determined specifications for identity, potency, purity, traceability and safety (Table 2). Accordingly, each batch of PEIpro®-HQ is tested and comes with detailed documentation on batch production and Quality Controls. This guarantees its use as a qualified raw material in cGMP processes and provides confidence to clinical investigation teams and regulatory authorities regarding batch-to-batch consistency, comparability, quality and traceability.
| Table 2: PEIpro®-HQ is a highly qualified grade of PEIpro® reagent. | |||
|---|---|---|---|
| PEIpro® | PEIpro®-HQ | ||
| Characteristics | |||
| 1 mg/ml of linear PEI in water | ✓ | ✓ | |
| Fully synthetic | ✓ | ✓ | |
| Manufacturing process | Identical | ||
| Quality controls | |||
| Identity | Molecular weight of intermediate product | ✓ | ✓ |
| Side chain content | ✓ | ||
| Assay | ✓ | ||
| Color, clarity | ✓ | ||
| pH | ✓ | ||
| Purity | Impurity profile | ✓ | |
| Safety | Endotoxin assay | ✓ | ✓ |
| Sterility test | ✓ | ✓ | |
| Mycoplasma | ✓ | ✓ | |
| Potency | Activity test | ✓ | ✓ |
| Documentation | |||
| Certificate of analysis | ✓ | ✓ | |
| Certificate of origin (confirming the absence of components of animal origin) | ✓ | ✓ | |
| Detailed batch production documentation to include in an IND or IMPD | ✓ | ||
| The quality of PEIpro® and PEIpro®-HQ are continuously assessed during the manufacturing process with suitable control testing. In comparison to PEIpro®-HQ, a more extensive number of Quality Controls are performed on both the bulk material and the formulated product to assess identity, purity, safety and potency of PEIpro®-HQ. | |||
Supporting viral vector manufacturing
To support growing viral vector-based commercialization demands, it is essential for all stakeholders in the supply chain to continually establish innovative solutions for improved viral vector manufacturing capacity and higher quality grade Final Drug Product. Offering a unique commercially available PEI-based transfection reagent that can support academics and biopharmaceutical companies from small- to large-scale clinical virus production is a crucial part of that effort.
Financial & competing interests disclosure
The authors are employees of Polyplus Transfection. No writing assistance was utilized in the production of this manuscript.
References
1. Ramello MC, Haura EB, Abate-Daga D. CAR-T cells and combination therapies: What’s next in the immunotherapy revolution? Pharmacol. Res. 2017.
CrossRef
2. Shaberman B. A Retinal Research Nonprofit Paves the Way for Commercializing Gene Therapies. Hum. Gene Ther. 2017; 28(12): 1118–21.
CrossRef
3. Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Methods Clin. Dev. 2016; 3: 16017.
CrossRef
4. Van der Loo JCM, Wright JF. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet. 2015; 25(R1): R42–52.
CrossRef
5. Kuroda H, Kutner RH, Bazan NG, Reiser J. Simplified lentivirus vector production in protein-freemedia using polyethylenimine-mediated transfection. J. Virol. Methods. 2009; 157: 113–21.
CrossRef
6. Hildinger M, Baldi L, Stettler M, Wurm FM. High-titer, serum-free production of adeno-associated virus vectors by polyethyleneimine-mediated plasmid transfection in mammalian suspension cells. Biotechnol. Lett. 2007; 29: 1713–1721.
CrossRef
7. Powers AD, Piras BA, Clark RK, Lockey T, Meagher MM. Development and optimization of AAV hFIX particles by transient transfection in an iCELLis fixed bed bioreactor. Hum. Gene Ther. Methods 2016; 27(3): 112–21.
CrossRef
8. Milian E, Julien T, Biaggio R et al. Accelerated mass production of influenza virus seed stocks in HEK-293 suspension cell cultures by reverse genetics. Vaccine 2017; 35, 3423–30.
CrossRef
9. Venereo-Sanchez A, Gilbert R, Simoneau M. Hemagglutinin and neuraminidase containing virus-like particles produced in HEK-293 suspension culture: An effective influenza vaccine candidate. Vaccine 2016; 34(29): 3371–80.
CrossRef
10. Finsinger D, Remy JS, Erbacher P, Koch C, Plank C. Protective copolymers for nonviral gene vectors: synthesis, vector characterization and application in gene delivery. Gene Ther. 2000; 7: 1183–92.
CrossRef
11. Boussif O, Lezoualc’h F, Zanta MA et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA 1995; 92: 7297–301.
CrossRef
12. Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999; 16: 1273–9.
CrossRef
13. Valkama AJ, Leinonen HM, Lipponen EM et al. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2017.
CrossRef
Affiliations
Alengo Nyamay’Antu1, Valérie Kedinger2, Patrick Erbacher3
1 Alengo Nyamay’Antu (Ph.D), Scientific & Technical Support Specialist, Polyplus Transfection
2 Valérie Kédinger (Ph.D), Clinical Key Account Manager, Polyplus Transfection
3 Patrick Erbacher (Ph.D), CSO, Polyplus Transfection
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</