Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study
Cell Gene Therapy Insights 2017; 3(10), 853-871.
10.18609/cgti.2017.082
Mesenchymal stromal cells (MSCs) are prime candidates for regenerative medicine and therapeutic applications due to both their potent immunomodulatory function and a unique ability to proliferate and differentiate into a variety of cell lineages. However, the stresses incurred during biopreservation/stability intervals (non-frozen and cryopreserved), including transit to and from the clinic can render MSCs ineffective and potentially unsafe. Challenges related to the formulation, transportation, distribution and delivery of source material (tissue, blood, marrow) and MSC-based products are important and inter-related components of the supply chain and scale-up. Effective biopreservation can optimize the quality of cell/tissue source material and final cell/tissue products, mitigate the adverse effects of interruptions and unforeseen events throughout the supply chain, as well as support the return to function of MSCs following patient administration. Conversely, inadequate environmental controls and biological support throughout the supply chain can limit transportation options, restrict the geographic distribution and reduce the clinical efficacy of MSC-based therapies. Indeed, it is possible that the contradicting reports in the literature on the impact of biopreservation on MSCs may stem from the lack of appropriate biopreservation protocols. Optimized biopreservation considerations are critical components of cell and tissue manufacturing systems, a robust and risk-mitigated supply chain, and are recommended for the commercialization of MSC-based products. This article aims to discuss the importance of Biopreservation Best Practices in the commercialization of MSC-based therapies and the relative benefits and concerns of different supply chain models.
Introduction
The diverse therapeutic attributes of mesenchymal stromal cells (MSCs) lend to their rapid development as cellular therapeutics. These unique attributes include potent immunomodulatory properties [1,2], the potential to differentiate into multiple cell lineages [3,4] and the ability to secrete myriad cytokines and exosomes [5], which can be exploited for developing treatments for GvHD [6,7], wound healing [8], tissue regeneration [9], acute trauma care [10,11] and other clinical applications [12,13]. Further to their therapeutic attributes, MSCs can be readily sourced from various tissues [14–16], demonstrate a high ex vivo proliferation capacity, and are naturally hypoimmunogenic, due to both a lack of MHC-II complex and co-stimulatory domains and the secretion of anti-inflammatory cytokines [17]. Collectively, these attributes make MSCs highly suitable for developing allogeneic, off-the-shelf therapies with the potential for large-scale commercial manufacturing. Nonetheless, there are practical challenges in the commercialization of MSC-based therapies including ex vivo cellular instability, which imposes logistical concerns surrounding Point-of-Care delivery. The incorporation of Biopreservation Best Practices is a commercialization imperative to secure the supply chain for MSC-based therapies, and process steps should be carefully designed to minimally impact MSCs’ viable recovery and return to function post-storage.
Cryopreservation is the established modality for the long-term preservation of biologics. Where the logistics of clinical laboratory work and transplantation/transfusion are uncertain, cryopreservation affords ample time for planning and manipulation. Commonly used ‘preservation’ media consist of an extracellular-like solution such as cell growth/culture media, saline or other physiologic buffers. Often, additional components such as serum and protein are added to these carrier solutions for osmotic support, while a cryoprotective agent (i.e., DMSO, glycerol, etc.) is supplemented when long-term storage via cryopreservation is warranted. Hypothermic preservation (traditionally on ice or at 2–8°C range) is an established biopreservation method, which slows biological degradation during a short-term ex vivo or culture excursion. Hypothermic storage is commonly employed when cells are intended for immediate processing or administration, when the logistical demands of utilizing frozen samples are limiting, or when cryopreservation damage in certain cell types exceeds the critical level for cell product efficacy.
Under normothermic conditions, the cells maintain a specific, cross-membrane gradient of ions through the ATP-driven action of membrane pumps. The tightly regulated intracellular ionic balance provides a platform for proper intracellular cell signaling, any alterations to which can have grave consequences on cell fate. During hypothermic storage and cryopreservation, increased membrane leakage combined with cold-induced dysfunction of membrane pumps, result in perturbation of intracellular ionic balance that can initiate apoptotic and necrotic pathways. A strategy to minimize cold-induced ionic perturbations is to maintain cells in a media that mimics the ionic balance of the intracellular milieu. Incorporation of such an ‘intracellular-like’ solution during the preservation period can minimize cold stress and associated downstream adverse cellular events.
Two major supply chain strategies are frozen and fresh delivery models, each with a set of requirements for trained staff and infrastructure. To select an optimal supply chain strategy, an awareness of the caveats to each preservation method is essential. In this case study, a commercially available, well-characterized, strain of MSCs was utilized as a representative cell model to investigate the potential impact of hypothermic storage and cryopreservation on MSC-based cell products. The results of this study can be used toward selecting an optimal supply chain strategy, which includes storage, transport and shipping practices for commercialization of cell-based products.
Materials & Methods
Cell culture
Human MSCs (hMSCs) were obtained from Lonza (MD, USA). Stock cultures were maintained at 37°C and 5% CO2 in Falcon T-75 cm2 flasks (VWR, PA, USA). HMSC cultures were grown in MSC basal medium (MSCBM) supplemented with MSCGM SingleQuots (Lonza, CA, USA). Stock cultures were subcultured every 5–6 days at approximately 95% confluence, and media was replenished every 3 days. Experiments were performed using cell cultures between passages 2 and 10. Prior to experiments, cultures were supplemented with fresh culture media for 1 day.
Differentiation of hMSCs to osteoblasts was performed according to manufacturer instructions (Lonza, MD, USA). Once differentiated, osteoblasts were maintained at 37°C and 5% CO2 in Falcon T-75 cm2-flasks in hMSC osteogenic differentiation medium.
Hypothermic storage
A variety of solutions were tested for hypothermic storage efficacy. In the extracellular-like category, the following solutions were tested: MSCBM (supplemented with 10% serum), Normosol®-R and Plasma-Lyte A (provided by Puget Sound Blood Center, now Bloodworks Northwest, WA, USA), AQIX (AQIX Ltd, UK), and Celsior® (Genzyme, MA, USA). For the intracellular-like solution category, ViaSpan (Barr Pharmaceuticals, NJ, USA) and HypoThermosol® FRS (HTS-FRS, BioLife Solutions, WA, USA) were tested.
In hypothermic storage experiments, cells were plated in 96-well culture plates and were grown to confluence (~104 cell/well). At this point, the media was replaced with the respective preservation solutions (100 µl/well), the plates were sealed with parafilm and were stored at 2–8°C for 1–7 days. Following hypothermic storage, plates were removed from cold, and the biopreservation solutions were replaced with serum-supplemented MSCGM, and the cells were allowed to recover under culture conditions for 1 day prior to assessment.
Cryopreservation
For cryopreservation studies, the following solutions with extracellular-like formulation were used: fetal bovine serum (FBS), MSCGM, Normosol-R and Plasma-Lyte. The cryopreservation media were prepared by supplementing these solutions with DMSO at 2, 5 or 10% v/v with or without 10% v/v FBS. The intracellular-like solutions for the cryopreservation experiments include CryoStor CS2, CS5 and CS10 (BioLife Solutions, WA, USA), which were all serum-free and protein-free, and were used as provided without further manipulation.
Human MSCs were grown to ~70–80% confluence in T-75 cm2 flasks, at which point they were detached by 10 min incubation with TrypLE® (GIBCO, Thermo Fisher Scientific, MA). After addition of 10x volume of MSCGM supplemented with 10% v/v FBS to neutralize TrypLE, centrifugation at 100 g, and removal of supernatant, the cell pellets were resuspended in respective cryopreservation solutions to achieve 6.0 x 105 cells/ml density and 0.5 ml of cell suspensions were placed into 1.2 ml cryovials. Vials were then placed inside a pre-chilled isopropyl alcohol passive freezing container. The samples were stored for 10 mins at 2–8°C to allow cell samples to equilibrate, then transferred to -80°C with manual ice nucleation at 20 mins after transfer. After 3 hours, frozen vials were transferred to LN2 for 18–24 hours. Samples were thawed in a 37°C water bath, immediately resuspended in culture media (1:10 dilution), plated, and allowed to recover in culture for 1 day prior to assessment. The control group consisted of non-cryopreserved cells.
Post-thaw hold time
To assess the impact of post-thaw hold time (non-frozen stability) on cell viable recovery, hMSCs were cryopreserved, as described above, in two groups: one group was cryopreserved in CryoStor CS10, and another group in Plasma-Lyte A supplemented with 10% v/v serum and 10% v/v DMSO (PL10). After thaw, each group of cryopreserved hMSCs was stored for 1 hour and 6 hours at refrigerated (2–8°C) and ambient (20–25°C) temperatures. After the post-thaw hold period, cells were returned to culture conditions for 24 hours before viable recovery was assessed by alamarBlue® metabolic activity assay. The control groups were immediately returned to normothermic culture conditions post-thaw.
Cell viability assessment
Cell viable recovery following the various treatments was assessed both qualitatively and quantitatively. The assessment was performed 1 and 3 days post-preservation for each experiment. Qualitative assessment was achieved by visualization using light microscopy. Quantitative assessment was accomplished using alamarBlue® (AbD Serotec, Bio-Rad, CA, USA). AlamarBlue® was diluted 1:20 in Hank’s Balanced Salt Solution (Life Technologies, MD, USA) without phenol red (HBSS). Culture medium from 96-well plates was removed and 100 µl of the working alamarBlue® solution was added to each well. Samples were then incubated in the dark at 37°C for 60 mins (±1 min). Fluorescence was evaluated using a Tecan SPECTRAFluorPlus plate reader (TECAN Austria GmbH, Austria) with a 530-nm excitation/590-nm emission filter set.
Fluorescence microscopy
Images of fluorescently-labeled cells were taken 1 day post-preservation. For staining, mitochondria were labeled with the cell-permeant MitoTracker Red CMXRos (final concentration of 500 nM), Alexa Fluor 488 phalloidin (1 unit/sample) was used to stain F-actin, and Hoechst 33342 (1 µg/ml) was used for staining cell nuclei (Invitrogen, CA, USA). In brief, culture media was removed from culture wells and a working solution of MitoTracker Red was prepared in culture media and was added at 50 µl/well. Cultures were incubated for 15 min at 37°C. Following incubation, solutions were removed and culture wells were washed gently twice with 10x diluted universal buffer (UB; Electron Microscopy Sciences) solution (1.5 M NaCl, 200 mM Tris, 0.1% NaN3, pH to 7.6 with HCl) with WFI-quality water. Cells were then fixed and permeabilized by adding 50 µl/well of fixative (3.7% formaldehyde in PBS) for 7 min, washed twice, permeabilized (0.2% Triton X-100 in 10x diluted UB) for 2.5 min, and washed twice. A working stock of phalloidin and Hoechst was prepared in 10x diluted UB and 50 µl/well was added to each well and incubated for 15 min at 37°C. Samples were washed twice with 10x diluted UB and 50 µl/well of solution was left in each well to prevent drying. Samples were then imaged at 20x magnification using a Zeiss Axiovert 200 fluorescent microscope with the AxioVision 4.7.2 software (Zeiss, Germany). At least three separate experiments were evaluated.
Mitochondrial morphology
Mitochondrial morphology was performed on epifluorescence images of MitoTracker Red CMXRos acquired using a Zeiss Axiovert 200 fluorescent microscope with the AxioVision 4.7.2 software (Zeiss, Germany) and a 20x LWD objective (568/595 nm excitation/emission). Images were processed using the Fiji/ImageJ software [18] as previously described [19]. Gray scale images were processed using convolve and median filters, respectively, followed by thresholding. Images were then subjected to particle analysis to determine mitochondrial area (A), perimeter (P) and aspect ratio (AR). Form factor (F) was calculated using the following equation:
![]()
Data analysis
Fluorescence units were converted to percent survival based upon experimental non-preserved controls (37°C). Calculations of standard error of the mean (SEM) were performed and statistical significance was determined using single factor ANOVA analysis. Cell viability experiments were repeated a minimum of three times with an intra-experimental repeat of 8 (n = 24).
Results
The impact of media formulation on the recovery of hMSCs following hypothermic storage
Hypothermic storage of hMSCs may be employed for short-term in vitro/ex vivo transportation between the patient/donor and the processing laboratory or manufacturing facility. To compare the efficacy of extracellular-like versus intracellular-like storage media on hMSC recovery after hypothermic storage, a variety of solutions, including intracellular-like HTS-FRS and ViaSpan, and extracellular-like MSCGM, AQIX, Plasma-Lyte, and Normosol-R were tested. After 3 days of storage in hypothermic conditions, hMSCs stored in HTS-FRS demonstrated robust morphology as indicated by intact actin cytoskeleton, and maintained a visually unbroken cell monolayer (Figure 1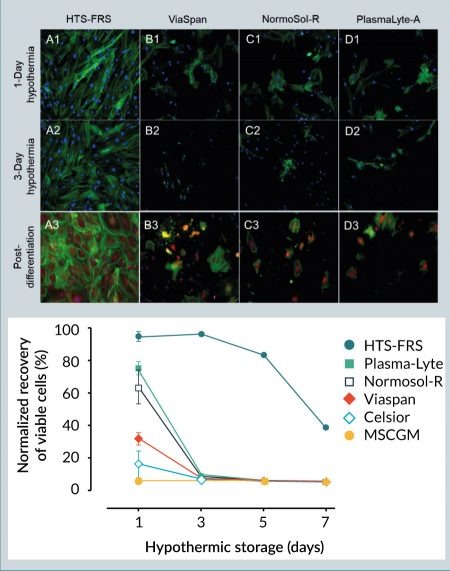
Mitochondria are the predominant site of cellular metabolism whose primary role is to convert substrates into stored cellular energy in the form of adenosine triphosphate (ATP). For decades considered merely static structures, mitochondria are increasingly recognized as dynamic organelles whose physical organization ranges from a punctate, globular appearance to an elongated, reticulated network. The general consensus is that fragmented (i.e., globular) mitochondria are indicative of mitochondrial dysfunction, diminished ATP production and an increase in the generation of deleterious reactive oxygen species, whereas a reticulated mitochondrial network is associated with enhanced ATP production, reduced oxidant generation, and a resistance to cellular stress [20]. Under normothermic conditions, undifferentiated hMSCs exhibited a predominantly reticulated mitochondrial appearance as revealed by image analysis of the mitochondrial ultrastructure [19] using the cationic fluorophore MitoTracker Red (Figure 2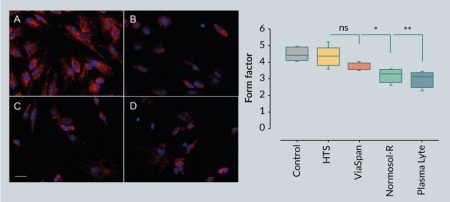
A reticulated mitochondrial appearance in hMSCs is consistent with previous reports [21,22]. Following 1 day of hypothermic storage, MitoTracker Red fluorescence generally decreased in hMSCs stored in the intracellular-like media ViaSpan, as well as those cells stored in the extracellular-like Normosol-R and Plasma-Lyte A, which is indicative of diminished mitochondrial function and consistent with a loss in cell viability (Figure 1). In addition, the extracellular-like media formulations resulted in significant alteration in mitochondrial morphology from a reticulated to a fragmented network as illustrated by a reduction in the mitochondrial form factor. By contrast, hypothermic storage in HTS-FRS conveyed a protective effect to hMSCs, as shown by robust MitoTracker Red staining and a more normal mitochondrial morphology. Despite the reduction in MitoTracker Red fluorescence in hMSCs stored in ViaSpan, hypothermic storage did not significantly impact mitochondrial morphology, suggesting that an intracellular-like storage solution protected mitochondrial function in those cells that survived the hypothermic insult.
Post-thaw recovery of cryopreserved hMSCs
Cryopreservation is currently the only option for long-term storage of living cells and tissues, with dimethyl sulfoxide (DMSO) being the most common cryoprotectant used to minimize freezing injury. To investigate whether the protective effects of an intracellular-like formulation extended to cryogenic storage, a standard extracellular-like cell culture growth media (MSCBM) supplemented with 10% serum was compared to intracellular-like, serum-free and protein-free CryoStor media. The optimal DMSO concentration for hMSC cryopreservation was determined by supplementing freezing media with 2, 5 and 10% v/v DMSO. After 24 hours post-thaw, hMSCs cryopreserved in MSCGM demonstrated a significant increase in viability from 23±1% to 65±3% with increasing DMSO content from 2 to 5%, and reached the highest post-thaw viable recovery at 72±3% with 10% v/v DMSO (Figure 3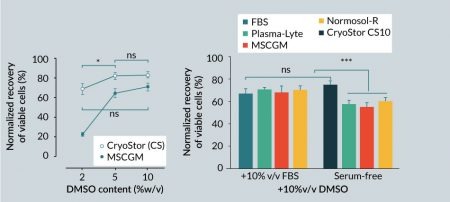
To assess the impact of serum on post-thaw viable recovery of hMSCs, different carrier solutions with extracellular-like formulations were supplemented with 10% DMSO and used to cryopreserve hMSCs in the presence or absence of 10% v/v serum. One group of cells was cryopreserved in full serum supplemented with 10% DMSO, and another group was cryopreserved in CryoStor CS10 to represent a serum-free, intracellular-like condition. Overall, serum-free media, with the exception of CryoStor CS10, resulted in lower post-thaw viable recovery compared to media supplemented with serum (Figure 3B). Average post-thaw cell viable recovery in serum-free media with 10% v/v DMSO was 75±3% for CS10, 55±4% for MSCGM, 60±3% for Normosol-R, and 57±3% for Plasma-Lyte A. In comparison, cell viability in the serum-supplemented group was 67±6% for MSCGM, 70±3% for Normosol-R, and 70±2% for Plasma-Lyte A. The DMSO-supplemented serum-only group resulted in 68±4% viability. Interestingly, although statistically equivalent to the serum-supplemented group, the average post-thaw viable recovery of hMSCs in serum-free CryoStor CS10 was the highest among all the groups. These results suggest that serum is not an essential isolated component for successful cryopreservation of hMSC, and a serum-free intracellular-like formulation can achieve similar or better post-thaw recovery at equivalent DMSO contents.
Effect of post-thaw holding time & temperature on recovery of cryopreserved hMSCs
It is often believed that cryopreserved cells should be immediately washed post-thaw to reduce the toxic effects of DMSO (or other cryoprotective agents). However, it may not be practical to incorporate an immediate wash step into all cell manufacturing processes, and the potential cell loss and/or cell damage due to wash/centrifugation manipulation steps may not be acceptable or optimal. As such, cells may be held in cryopreservation media in a non-frozen state, and thus exposed to DMSO for an extended period of time post-thaw in clinical or manufacturing settings. To evaluate the effect of post-thaw non-frozen hold time (i.e., post-thaw stability) on viability, hMSCs cryopreserved in CryoStor CS10 or Plasma-Lyte supplemented with 10% v/v serum and 10% v/v DMSO (PL10) were held for 1 hour and 6 hours post-thaw under refrigerated (2–8 °C) and ambient/room (20–25 °C) temperatures. In general, despite the observed trends, the 2-way ANOVA analysis concluded the differences were not statistically significant, potentially due to the small sample size (Figure 4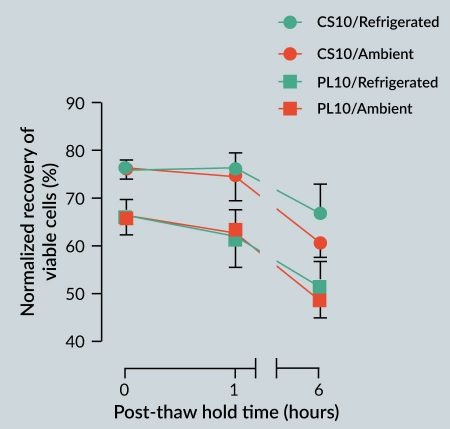
It was interesting to note that, regardless of the storage temperature, the average viable recovery of the hMSCs cryopreserved in CS10 or PL10 did not significantly change up to 1 hour post-thaw. This finding suggests a minimal short-term toxicity to hMSCs from DMSO and other components of the respective cryopreservation media. It should also be noted that 6 hours post-thaw refrigerated storage of cells cryopreserved in CS10 resulted in slightly higher viabilities when compared to storage at ambient storage. The similar trend between the CS10 and PL10 groups at both temperatures suggests that the loss of viability in both groups is likely due to DMSO toxicity. Nonetheless, the 6-hour post-thaw viable recovery of the cells preserved in CS10 was statistically equivalent to the immediate post-thaw viable recovery of the cells that were cryopreserved in PL10. These results suggest that an intracellular-like carrier formulation with improved ionic balance can extend the time in which hMSCs can be exposed to DMSO without a significant negative impact on viable recovery, and as such, offers a more robust cryopreservation option for large-scale cell manufacturing.
Discussion
Biopreservation is an integral part of the supply chain of any commercially viable cellular therapy, as well as clinical center cellular therapies that are increasingly impacted by time and distance. Biopreservation refers to the processes that support the stability of biological cells to ensure a return to function post-preservation. Traditionally, this is achieved by lowering the temperature to just above the freezing point of water (2–8°C) to slow down cellular metabolism, or by freezing cells to cryogenic temperatures (such as liquid nitrogen, -196°C) to arrest all biological activities in a suspended animation state. However, in both cases, cold-induced physical and physiological stresses can accumulate to an extent that harms the cells beyond the capacity to repair, triggering downstream apoptotic and secondary necrotic cell death pathways. Optimized biopreservation strategies reduce such cold-induced stresses and can improve cellular health and functionality following storage. With respect to commercialized cell and gene therapies, effective biopreservation protocols can provide much needed flexibility in manufacturing and shipping, facilitate effective process development and reduce manufacturing costs.
Infrastructure considerations for manufacturing & Point-of-Care, & the implications of selecting the optimal biopreservation strategy
The choice of supply chain model for commercially manufactured cellular therapies influences the infrastructure needs at the Point-of-Care (PoC). For transportation to a central manufacturing facility or the PoC, there are two primary models for the supply chain: namely, frozen delivery and thaw at the destination, and (2) formulate for fresh (non-frozen) delivery (Figure 5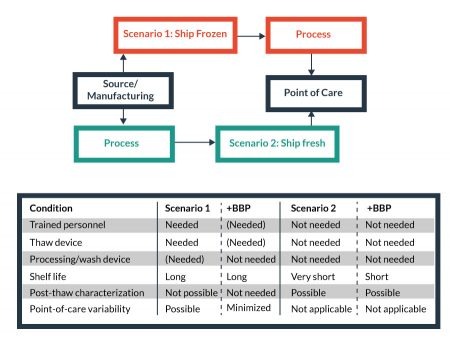
For example, the frozen model requires trained personnel and proper equipment at the PoC, while such needs are reduced in the fresh delivery model. On the other hand, fresh delivery, with acute shelf-life limitations, is more vulnerable negative logistical impacts than a frozen delivery model. Some reduced costs of non-frozen transport (i.e., lack of LN2 dry shipper or equivalent, weight, etc.) may be offset by the needs of faster delivery times and more stringent logistics controls to facilitate a smaller window of delivery time to maintain cell product stability. Whether frozen or fresh, enabling Biopreservation Best Practices can be beneficial to the processing-related costs, and facilitate the development of broadly available cellular products. For this purpose, the evaluation and/or validation of optimal biopreservation parameters for both short-term (hypothermic) and long-term (cryopreserved) storage for each cell product is recommended.
Hypothermic storage of hMSCs
It is not always feasible to immediately process and cryopreserve the cells following harvest and isolation, or upon receiving for patient delivery. As cells gradually degrade following removal from the human body or culture conditions, transportation of the cells can adversely impact viable recovery and function. In addition, cell thawing at the PoC can be a point of risk and variability for the utilization of cryopreserved products. Alternatively, hypothermic storage requires minimal manipulation steps, and eliminates the need for specialized freezing and thawing equipment at the point of collection or administration, and can be considered as an option for the delivery of fresh (non-frozen) cells that may be directly administered at the PoC without further processing.
Numerous reports have documented that refrigerated storage can facilitate an improvement in overall recovery and stability in multiple cell types (including stem cells) versus storage at room temperature [23–26]. However, significant loss of recovery generally occurs when cells are hypothermically stored beyond 24 hours [24,27]. In many cases, cells and tissues, including apheresis/leukapheresis collections, are transported from source location to a processing site over long distances, or may be delayed due to unforeseen logistic circumstances, including unplanned patient unavailability or transport service interruption. Under such circumstances, extended stability is of benefit for a cellular product intended for downstream processing or patient application.
In this study, we found that refrigerated storage of hMSCs in the intracellular-like, serum-free and protein-free HypoThermosol FRS (HTS-FRS) media can support maintaining nearly ~100% viable recovery of hMSCs up to 72 hours and can maintain high levels of viable recovery (~80%) up to 120 hours. Under similar storage conditions, all other media options tested in this study were unable to support the recovery of the cells at any level for more than 24 hours. The accumulation of cellular stresses induced by cold, as evidenced by disruption in the actin cytoskeleton and mitochondrial fission and granulation, can contribute to a loss of viability and recovery after 1 day of refrigerated storage. With slowed ATP generation and ion pump activity, cold storage in extracellular-like medium can cause an intracellular ionic imbalance, which leads to the disruption of normal cellular functions [28]. Interestingly, while the intracellular-like organ preservation media ViaSpan (also known as University of Wisconsin or UW solution) was not as effective for the recovery of viable cells, it was effective at maintaining mitochondrial ultrastructure in the cells that survived. We posit that the differences in hMSC viability between HTS-FRS and ViaSpan may be due to the differential osmolytes in HTS-FRS versus ViaSpan, while the reduction in mitochondrial stress could be due to the intracellular-like ionic concentrations in both formulations. The hypothermic storage and stability benefits afforded by HTS-FRS have been described in a number of cell and tissue applications [29–31], as well as its inclusion in a number of clinical applications [28,32,33]. The current study describes those benefits in an hMSC model, showing that surviving hMSCs can differentiate into other cells regardless of the biopreservation media tested (Figure 1). However, beyond 1 day of hypothermic storage, only the hMSCs stored in HTS-FRS were still viable and capable of differentiating into osteoblasts.
Cryopreservation of hMSCs
Preservation of cellular phenotype, including the expression of specific surface membrane proteins, is a vital component of therapeutic efficacy for clinical/commercial cell products. Several relatively recent reports have presented contradicting evidence regarding the impact of cryopreservation on the viability and functionality of hMSCs. On one hand, reports demonstrate that cryopreserved MSCs appeared to exhibit an impaired immunosuppressive potential and decreased engraftment capacity compared to freshly isolated cells [34,35]. Other studies have similarly reported on the compromised qualities of MSCs immediately post-thaw that can be reversed within 24–72 hours by post-thaw culture/recovery [3]. Such observations have led some researchers to believe that MSC cryopreservation, especially for certain applications, should be avoided or that cells must be rescued post-thaw and administered ‘fresh’. On the other hand, other findings attest to the robustness of hMSCs when cryopreserved using differing cryopreservation protocols [36,37]. In one such study by Yuan et al., the proliferation, differentiation capacity and expression of engineered surface proteins after cryopreservation remain comparable to freshly isolated controls [38]. As the cryopreservation protocols differ among the studies cited in this section, including whether utilizing extracellular-like cocktails or intracellular-like biopreservation media, the disparities in post-thaw viable recovery and function could be attributed to any number of variables related to the cryomedia composition and/or freezing protocol. In our study, commercially procured hMSCs were used as a cell model to investigate the impact of cold-induced stresses on viability and recovery post-thaw in commonly employed cryomedia formulations. However, hMSC characteristics vary between donor and source, and consequently, in-depth characterization and phenotypic stability was not a focus of this study. Investigation of phenotypic characteristics of a specific hMSC population likely vary between samples and is recommended to be validated for each application to ensure safe and effective cell-based therapies.
It is important to note that overlooking the principles of biopreservation in practice may result in inconsistent and contradictory outcomes such as those reported for post-thaw hMSC function [39,40]. The cryopreservation media employed in the cited studies are composed of an extracellular-like base media (including cell culture/growth media or physiologic buffers such as saline, plasma, Normosol-R and Plasma-Lyte A) supplemented with serum and/or protein and varying amounts of DMSO. An extracellular-like solution closely resembles the ionic composition of the human serum and interstitial fluid that is balanced for cells at normothermic temperature (37°C). When stored at reduced temperatures in extracellular-like solutions such as culture media or physiological saline, the delicate intracellular ionic balance can be significantly disturbed, and extracellular-like compositions are not balanced or buffered for these low temperature conditions. Cold-induced cellular shifts include: transient membrane permeability [41]; inactivation of ATP-driven ion pumps and osmotic swelling [42]; alteration of protein solubility and aggregation [43]; anaerobic metabolism that leads to lactate accumulation and a resultant intracellular pH shift [44,45]; and increased free radical generation and reduced capacity to scavenge free radicals [46,47]; all which can accumulate and culminate in the loss of cell yield, viability and function during and after storage. In the absence of Biopreservation Best Practices, which includes incorporation of an intracellular-like base media, the definitive attribution of hMSCs functional loss post-thaw to cryopreservation per se is questionable. Indeed, the present data suggests that improper cryopreservation protocols and media may be a major contributor to observed post-thaw functional loss. The results in this study reinforce the notion that hMSCs are exposed to freezing injury when cryopreserved in traditional isotonic/extracellular-like cryopreservation home-brew cocktails in the absence of serum (Figure 3B). However, such cryopreservation injury can be minimized using a base medium that mimics an intracellular-like ionic balance with relevant sugars and polysaccharides (sometimes termed osmolytes) as a serum-free and protein-free biopreservation solution. Such an intracellular-like formulation enabled post-thaw viable recovery similar to, or better than, serum-supplemented cryopreservation media (Figure 3A) consistent with previous reports [48–50]. While the results in Figure 3A suggest a potential correlation between DMSO concentration and post-thaw viability, it must be noted that viability reaches an optimum at around 5–10% DMSO, depending on cell type [38]. The kinetics of DMSO toxicity amplify with increasing concentration and may correlate with adverse events when delivered to patients [51], and as such, cryomedia containing DMSO at concentrations higher than 10% are rarely used in practice.
Broader implications of biopreservation best practices in the supply chain of cellular therapies: a perspective
Decentralized manufacturing processes, including thawing, isolation steps, washing and characterization, are undesirable burdens for commercialization of cellular therapies, resulting in potential increase in cost of goods (COGs). Nonetheless, such processes are common in most ongoing pre-clinical and clinical studies in the field of cell therapy and Regenerative Medicine. Implementing Biopreservation Best Practices early in the development phase of these studies not only may improve the quality of the final cell product in terms of viable recovery and function, but can also address an array of potential risks that include safety and regulatory aspects, manufacturing costs, and the robustness of the supply chain. Further, the presence of serum or protein in biopreservation media in manually prepared ‘home-brew’ cocktails can increase the risk of contamination, disease transmission, and formulation error, are not recommended and consistent with current Good Manufacturing Practices (cGMP) [39,52]. Cell-based manufacturing guidelines promote the manufacturing of cellular products in well-defined, serum-free and protein-free media. A final therapeutic product formulated with reduced risks may result in qualification for a ‘thaw-and-infuse’ application model, which can minimize the risk factors and costs associated with specialized processing and equipment at the PoC Minimizing post-thaw manipulations may lower the risk factors and costs of cellular therapies, and improve access to these promising therapies as they become more routine and transition from specialized centers to local clinics and physician offices.
Incorporation of Biopreservation Best Practices may convey other advantages such as increased stability post-thaw (Figure 4). Indeed, Yang et al. reported that cryopreserved hematopoietic progenitor cells exhibited a significant and progressive reduction in post-thaw viable nucleated cell count at temperatures of 22°C and 37°C [53]. For a number of reasons, the cellular product may need be stored for a limited time at the PoC under refrigerated or ambient conditions, both after thaw and before application. These reasons may include scheduled processing, cell wash steps and dose preparation, unplanned interruptions such as temporary patient or equipment unavailability, or other logistical considerations. Depending on the cell type and conditions, such situations can result in significant post-thaw loss of yield, viability and function, or even scrapped therapeutic product. For MSC-based therapies, the ability to introduce a post-thaw cell holding (stability) step without significant loss of cell quality reinforces the cryopreservation process against external/unpredictable variables, minimizes variability, and ensures desired viable cell recovery post-thaw [23,49].
Concluding Remarks
Biopreservation Best Practices ensure cells remain viable and functional throughout the supply chain and manufacturing workflow of the cell product, and convey Quality/Regulatory and Cost-of-Goods benefits toward clinical and commercial development of cell-based therapies. In this case study, we investigated short-term hypothermic storage and long-term cryopreservation options using an hMSC-representative model system. Our results demonstrate that traditional isotonic extracellular-like storage media (cell growth media, physiologic buffers and intravenous infusion fluids) were not the most effective options to support extended stability and maximize post-preservation viable recovery of hMSC. In contrast, the intracellular-like formulation of HypoThermosol FRS (HTS-FRS) improved overall viable recovery during hypothermic storage, and permitted extended storage for a minimum of 3 days. When cryopreserved, hMSCs exhibited the highest viable recovery in the intracellular-like CryoStor compared to alternative cryopreservation media with and without the addition of serum. In addition to a quantitative focus on post-preservation yield, viability and recovery of functional activity, the Quality and Regulatory footprint of biopreservation media should also be considered suitable for developing cellular therapies for clinical applications. Elimination of serum and protein that may introduce risk, while maintaining post-preservation yield, viability and function, facilitates the Quality and Regulatory materials qualification process. While a variety of home-brew cocktails, pre-formulated media and methods are available for storage of hMSCs, appropriate risk management considerations are recommended when qualifying the solutions for short-term and long-term biopreservation.
Financial & competing interests disclosure
Alireza Abazari, Brian J Hawkins and Aby J Mathew are employees of BioLife Solutions. Dominic M Clarke was an employee of BioLife Solutions at the time of data collection.
No writing assistance was utilized in the production of this manuscript.
References
1. Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes 2008; 57:1759–67.
CrossRef
2. Amorin B, Paula A, Vanessa A. Mesenchymal stem cell therapy and acute graft-versus-host disease : a review. 2014: 137–50.
3. Otsuru S, Hofmann TJ, Raman P et al. Genomic and functional comparison of mesenchymal stromal cells prepared using two isolation methods. Cytotherapy 2015; 17: 262–70.
CrossRef
4. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8(4): 315–7.
CrossRef
5. Caplan AI. MSCs: The Sentinel and Safe-Guards of Injury. J. Cell Physiol. 2016; 231: 1413–6.
CrossRef
6. Le Blanc K, Frassoni F, Ball L et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet (London, England) 2008; 371: 1579–86.
CrossRef
7. Engelhardt BG, Jagasia M, Savani BN et al. Regulatory T cell expression of CLA or (4)(7) and skin or gut acute GVHD outcomes. Bone Marrow Transpl. 2011; 46: 436–42.
CrossRef
8. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells 2007; 25: 2648–59.
CrossRef
9. Spitzer TLB, Rojas A, Zelenko Z et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype. Biol. Reprod. 2012; 86: 58–58.
CrossRef
10. Hasan A, Deeb G, Rahal R et al. Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol. 2017; 8.
CrossRef
11. Huber-Lang M, Wiegner R, Lampl L, Brenner RE. Mesenchymal Stem Cells after Polytrauma: Actor and Target. Stem Cells Int. 2016; 2016.
12. Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant 2016; 25: 829–48.
CrossRef
13. D’souza N, Rossignoli F, Golinelli G et al. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med. 2015; 13: 186.
CrossRef
14. Huang GT-J, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J. Dent. Res. 2009; 88: 792–806.
CrossRef
15. In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004; 22: 1338–45.
CrossRef
16. Sun L, Wang D, Liang J et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010; 62: 2467–75.
CrossRef
17. De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr. Mol. Med. 2012; 12: 574–91.
CrossRef
18. Schindelin J. Fiji: an open-source platform for biological-image analysis. Nat. Methods 2012; 9.
CrossRef
19. Koopman WJH, Visch H-J, Smeitink JAM, Willems PHGM. Simultaneous quantitative measurement and automated analysis of mitochondrial morphology, mass, potential, and motility in living human skin fibroblasts. Cytometry A 2006; 69: 1–12.
CrossRef
20. Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012; 46: 265–87.
CrossRef
21. Phinney DG, Di Giuseppe M, Njah J et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015; 6: 8472.
CrossRef
22. Li C-J, Chen P-K, Sun L-Y, Pang C-Y. Enhancement of Mitochondrial Transfer by Antioxidants in Human Mesenchymal Stem Cells. Oxid. Med. Cell Longev. 2017; 2017: 8510805.
23. Pal R, Hanwate M, Totey SM. Effect of holding time, temperature and different parenteral solutions on viability and functionality of adult bone marrow-derived mesenchymal stem cells before transplantation. J. Tissue Eng. Regen. Med. 2008; 2: 436–44.
CrossRef
24. Antonenas V, Garvin F, Webb M, Sartor M, Bradstock KF, Gottlieb D. Fresh PBSC harvests, but not BM, show temperature-related loss of CD34 viability during storage and transport. Cytotherapy 2006; 8: 158–65.
CrossRef
25. Basso N, Mirkopoulos P, Heersche JNM. Osteoprogenitor viability in cell populations isolated from rat femora is not affected by 24 h storage at 4 degrees C. Cryobiology 2005; 50: 211–5.
CrossRef
26. Pamphilon D, Curnow E, Belfield H et al. Storage characteristics of cord blood progenitor cells: report of a multicenter study by the cellular therapies team of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion 2011; 51: 1284–90.
CrossRef
27. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–7.
CrossRef
28. Buskirk RG Van, Baust JM, Snyder KK, Mathew AJ, Baust JG. Successful Short- and Long-Term Preservation of Cells and Tissues. Bioprocess Int. 2004; 42–9.
29. Mathew AJ. I’m Losing Cell Viability and Function at Different Points in My Process, and I Don’t Know Why! Bioprocess Int. 2010: 54–7.
30. Yang Y, Steeg J, Honaramooz A. The effects of tissue sample size and media on short-term hypothermic preservation of porcine testis tissue. Cell Tissue Res. 2010; 340: 397–406.
CrossRef
31. Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Preservation of rat livers by cold storage: a comparison between the University of Wisconsin solution and Hypothermosol. Ann. Transplant. 2004; 9: 35–7.
32. Povsic TJ, O’Connor CM, Henry T et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am. Heart J. 2011; 162.
CrossRef
33. Powell RJ, Comerota AJ, Berceli SA et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J. Vasc. Surg. 2011; 54: 1032–41.
CrossRef
34. François M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon- licensing. Cytotherapy 2012; 14: 147–52.
CrossRef
35. Chinnadurai R, Garcia MA, Sakurai Y et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Rep. 2014; 3: 60–72.
CrossRef
36. Kotobuki N, Hirose M, Machida H et al. Viability and Osteogenic Potential of Cryopreserved Human Bone Marrow-Derived Mesenchymal Cells. Tissue Eng. 2005; 11: 663–73.
CrossRef
37. Chin S-P, Poey AC, Wong C-Y et al. Cryopreserved mesenchymal stromal cell treatment is safe and feasible for severe dilated ischemic cardiomyopathy. Cytotherapy 2010; 12: 31–7.
CrossRef
38. Yuan Z, Lourenco SDS, Sage EK, Kolluri KK, Lowdell MW, Janes SM. Cryopreservation of human mesenchymal stromal cells expressing TRAIL for human anti-cancer therapy. Cytotherapy 2016; 18: 860–9.
CrossRef
39. Grein TA, Freimark D, Weber C, Hudel K, Wallrapp C, Czermak P. Alternatives to dimethylsulfoxide for serum-free cryopreservation of human mesenchymal stem cells. Int. J. Artif. Organs 2010; 33: 370–80.
40. Hanna J, Hubel A. Preservation of stem cells. Organogenesis 2009; 5: 134–7.
CrossRef
41. Hays LM, Crowe JH, Wolkers W, Rudenko S. Factors affecting leakage of trapped solutes from phospholipid vesicles during thermotropic phase transitions. Cryobiology 2001; 42: 88–102.
CrossRef
42. Boutilier RG. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001; 204: 3171–81.
43. Long FA, McDevit WF. Activity Coefficients of Nonelectrolyte Solutes in Aqueous Salt Solutions. Chem. Rev. 1952; 51: 119–69.
CrossRef
44. Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J. Cereb. Blood Flow Metab. 2003; 23: 513–30.
CrossRef
45. Pulis RP, Wu BM, Kneteman NM, Churchill TA. Conservation of phosphorylation state of cardiac phosphofructokinase during in vitro hypothermic hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2000; 279: H2151–8.
46. Guzy RD, Hoyos B, Robin E et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005; 1: 401–8.
CrossRef
47. Alva N, Palomeque J, Carbonell T et al. Oxidative stress and antioxidant activity in hypothermia and rewarming: can RONS modulate the beneficial effects of therapeutic hypothermia? Oxid. Med. Cell Longev. 2013; 2013: 957054.
48. Malpique R, Ehrhart F, Katsen-Globa A, Zimmermann H, Alves PM. Cryopreservation of adherent cells: strategies to improve cell viability and function after thawing. Tissue Eng. Part C Methods 2009; 15: 373–86.
CrossRef
49. Clarke DM, Yadock DJ, Nicoud IB, Mathew AJ, Heimfeld S. Improved post-thaw recovery of peripheral blood stem/progenitor cells using a novel intracellular-like cryopreservation solution. Cytotherapy 2009; 11: 472–9.
CrossRef
50. Stylianou J, Vowels M, Hadfield K. Novel cryoprotectant significantly improves the post-thaw recovery and quality of HSC from CB. Cytotherapy 2006; 8: 57–61.
CrossRef
51. Rowley SD, Feng Z, Yadock D, Holmberg L, MacLeod B, Heimfeld S. Post-thaw removal of DMSO does not completely abrogate infusional toxicity or the need for pre-infusion histamine blockade. Cytotherapy 1999; 1: 439–46.
CrossRef
52. Thirumala S, Goebel WS, Woods EJ. Clinical grade adult stem cell banking. Organogenesis 2009; 5: 143–54.
CrossRef
53. Yang H, Acker JP, Cabuhat M, McGann LE. Effects of incubation temperature and time after thawing on viability assessment of peripheral hematopoietic progenitor cells cryopreserved for transplantation. Bone Marrow Transpl. 2003; 32: 1021–6.
CrossRef
Affiliations
Alireza Abazari1, Brian J Hawkins1,2, Dominic M Clarke3 & Aby J Mathew1*
1 BioLife Solutions, Inc., Bothell, WA, USA
2 University of Washington Seattle, Seattle, WA, USA
3 Charter Medical Ltd., Winston-Salem, NC, USA
* Corresponding author: amathew@biolifesolutions.com
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</