Personalized Supply Chains for Cell Therapies
Cell Gene Therapy Insights 2017; 3(10), 815-833.
10.18609/cgti.2017.081
The commercialization of two recent autologous genetically engineered T-cell oncology products was a monumental moment for the cell therapy industry and patients. These market approvals, however, have jointly reinforced the need for technological solutions to maintain the complex supply chains of the industry. These supply chains, which are patient-centric, involve multiple stakeholders, including patients, providers, collection centers, couriers, case managers and the manufacturers, all of which must be orchestrated properly for success. Systems currently used by drug manufacturers, such as enterprise and manufacturing systems, quality management systems and patient management systems, although essential, do not provide the needle-to-needle traceability and precise orchestration of the supply chain. To support the requirements of these unique supply chains, cloud based, configurable platforms called Cell Orchestration Platforms (COP) have been designed to both schedule and manage stakeholders and events while also maintaining chain of identity and custody. COPs are not intended to replace existing core systems but are intended to integrate with them to provide a connected end-to-end view of the supply chain. This article aims to provide insight into the function and implementation strategy for COPs, while also considering their strategic position and connectivity with other adjacent systems within the autologous cell therapy supply chain. Additionally, the article reviews the roles of key supply chain stakeholders and the systems they interact with to ultimately provide a seamless experience for physicians and patients.
August 30th and October 18th of this year were momentous days in medicine. Novartis’s Kymriah™ and Kite Pharma’s Yescarta™ were approved by the US FDA for the treatment of acute lymphoblastic leukemia (ALL) and aggressive non-Hodgkin lymphoma (NHL), respectively, making them the first CAR-T therapies to reach the market. On the heels of Kymriah™ and Yescarta™ are a considerable number of additional immunotherapy companies pushing clinical development of their own products. As the majority of these companies are developing autologous, or patient-specific therapies, a unique manufacturing event is required for each patient – unlike traditional drug development where drug products are made in large batches, yielding thousands of units per campaign (outlined further in Table 1). The efforts to manage these complex patient-centric supply chains are considerable, necessitating teams of dedicated human resources even during early-stage clinical trials when patient numbers are small. As patient numbers increase into the hundreds during pivotal trials and dozens of clinical sites begin to extend across multiple countries, this makes electronic systems engineered to orchestrate the activities and precise timing of events across the supply chain imperative, and a requirement for commercial approval.
| Table 1: Comparison of monoclonal antibody and autologous immunotherapy supply chains. | |
|---|---|
| Monoclonal antibody | Autologous cell therapy |
| Simple decision-to-treat process if product/pricing approved by payers | Decision-to-treat likely to require case-by-case assessment of funding due to high product cost to payers |
| Starting materials well categorized | Starting material has high variability and may have a short shelf life |
| Single or limited number of GMP-compliant sources for active pharmaceutical ingredient (API) | Multiple sources of starting material (apheresis centers) – potential for inconsistent processes |
| Single lot = 1000s of patients; make-to-stock is possible | Single lot = 1 patient; therapy manufactured in real time |
| Product is not personalized | Needle-to-needle traceability required – patient must receive therapy manufactured from own cells |
| Long and large clinical trials – long-term safety/efficacy data; time to build global experience and plan commercial supply chains | Short and small clinical trials – limited data means long-term safety studies may be required; less time to plan and develop commercial supply chain |
| Simpler reimbursement models based on massive amount of commercialization experience | Reimbursement models potentially more complex and based on outcomes |
| Short-term follow-up if treatment is successful | Long-term follow-up (~15 years), even if treatment is successful or curative |
| Can be scaled up without scaling out | Scale-up only achieved by scaling out, increasing supply chain complexity |
| Mature, established and standardized high-volume transportation lanes from manufacturing site to distributor using large integrator couriers | Less established transportation lanes using specialized, high cost white glove couriers with fewer standardized processes |
| Critical quality attributes can be clearly defined | Process inputs (e.g., handling, environmental conditions) need to be tightly controlled |
Never before in the history of drug development has the supply chain and logistics been so deeply integrated and inherently connected to the success of a single patient treatment. Dendreon, an early pioneer of cell-based immunotherapy, recognized that logistics was a critical element and invested millions of dollars into its logistics orchestration system, branded Intellivenge™. As no commercial system existed a decade ago to manage the complexities of an autologous supply chain, Dendreon outsourced the build of its custom orchestration system [1]. In September 2009, the company announced that they were on track to complete the submission of their biologics license application amendment to the FDA by the middle of November [2]. This announcement detailed the company’s plan to have 120 work stations in place across its manufacturing centers in New Jersey, Georgia and California by 2011 [2]. Additionally, Dendreon stated that it expected to have contracts with 150–200 apheresis centers in the USA at peak demand [2]. Dendreon lastly announced that it had developed and will implement Intellivenge™, a first-of-its-kind advanced logistical and patient treatment management and planning system, which coordinates the patient and physician scheduling as well as the operational and logistic activities should Dendreon’s lead product, Provenge™, be approved by the FDA [2].
At the same time, across the Atlantic, a team of engineers at Swansea University, UK, teamed up with cell therapy and supply chain experts to develop an industry and regulatory compliant cell software platform to maintain a complete electronic record trail, ensuring chain of identity and chain of custody from collection of patient-specific starting material through to infusion of final drug product. In 2012, the efforts resulted in the birth of the first industrial-scale software platform specifically designed to manage the complexities of the autologous cell therapy supply chain, TrakCel. Clinical success and overall industry demand for such a system has resulted in a growing number of technology solutions now available on the market place.
In April of that same year (2012), a 7-year old cancer patient, Emily Whitehead, became the first child to be enrolled in CTL019 at Children’s Hospital of Philadelphia, a clinical trial for patients with ALL, NHL and chronic lymphocytic leukemia [3]. Four months later, Novartis announced an exclusive global research and licensing agreement with the University of Pennsylvania to further study and commercialize cellular immunotherapies using chimeric antigen receptor (CAR) technologies [4]. The first major genetically engineered immunotherapy competitors to Novartis – Kite Pharma and Juno Therapeutics – quickly followed. This marked the birth of the genetically engineered immunotherapy industry (Table 2).
| Table 2: Key milestone events in the commercialization of KymriahTM. | |
|---|---|
| Date | Novartis KymriahTM (CTL019) key event milestones |
| April 2012 | Emily Whitehead is the first child treated with CTL019 for ALL [3] |
| August 2012 | University of Pennsylvania and Novartis form an alliance to expand use of personalized T-cell therapy for cancer patients [4] |
| December 2013 | University of Pennsylvania reports on study of first 59 leukemia patients who received personalized cellular therapy [8] |
| July 2014 | FDA designated CTL019 as a Breakthrough Therapy under the Penn investigational new drug application [9] |
| December 2015 | Novartis highlights new CTL019 Phase 2 data demonstrating 93% complete remission in pediatric patients with ALL [10] |
| June 2017 | Novartis pivotal CTL019 6-month follow-up data shows durable remission rates in children, young adults with B-cell ALL [11] |
| August 2017 | Novartis receives first ever FDA approval for a CAR-T cell therapy, Kymriah™ (CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice [12] |
Companies developing autologous cell-based immunotherapies may in fact be equally in the business of logistics as they are in biology, especially as the technologies reach pivotal trials and commercialization. Furthermore, due to the intrinsic logistical requirements, early process development efforts will go well beyond studies to scale-up or scale-out the manufacturing process within the cleanrooms. These efforts will additionally focus on optimizing processes and electronic systems to manage the complex supply chain within these development efforts. As early pioneers, Dendreon and Novartis had no choice but to build their own logistics orchestration systems. The effort to build IT systems internally is not only incredibly time consuming and labor intensive but also involves significant capital and risk. Today, highly configurable and cost-effective commercial scale systems have been developed specifically for this industry – they have been termed Cell Orchestration Platforms (COP) and serve a critical role in the cell therapy supply chain.
The purpose of this review is to provide insight into the role and function of the COP and other adjacent systems within the autologous cell therapy supply chain, along with insights into the key supply chain stakeholders and their corresponding requirements for a successful scale-up and scale-out of cell therapy production to support eventual commercialization.
Supply Chain Orchestration Technologies
The primary function of a COP is to manage the multitude of events and maintain chain of identity and custody throughout the supply chain. Chain of identity allows each therapy to be tracked back to the original donor. This is critical for both autologous and allogeneic therapies as this tracking information will allow failed batches to be traced back to original source material. Chain of custody allows each therapy to be tracked through the supply chain in real time, providing users with a needle-to-needle dashboard view of each therapy’s progress. COPs also allow for the implementation of client- and product-specific workflows to ensure that each process step and corresponding data capture is conducted consistently across multiple stakeholders. This is essential for both upstream and downstream of the GMP manufacturing process to industrialize the process outside of the manufacturing facility. The scheduling of activities across multiple stakeholders should also be executed by the COP, which will help eliminate any process gaps and help maximize the utilizations of facility and human assets. And of course, throughout the entire supply chain, data will be captured for future analytics, which can help optimize the supply chain (Figure 1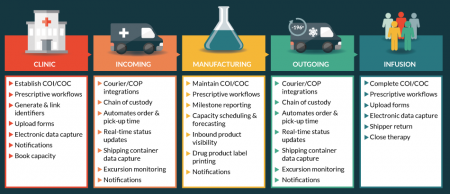
COP systems are not intended to replace existing systems, but are intended to integrate information from multiple other systems to provide clients with an end-to-end single-interface view of the supply chain. From a commercialization viewpoint, outside of the COP, the other crucial systems needed to support commercial-scale volumes are enterprise (ERP) and manufacturing systems (MES), quality management systems (QMS), patient management systems and electronic clinical outcome assessment (eCOA) systems. To allow for seamless management of all key information, integrations may be required between systems.
In addition to integrations with ERP/MES systems and QMS, manufacturers may also contract with a patient management company to handle patient issues, such as access, scheduling, adherence and reimbursement. These services are often delivered by assigned case managers that will work with patients and physicians. The communication and delivery of these services will be facilitated primarily through patient management systems.
Finally, integrations with electronic clinical outcomes assessment (eCOA) software may allow for the collection and compiling of essential outcome safety and efficacy data that can be used for many purposes including the trigger of key milestone reimbursement payments (discussed further in Payers section). Table 3 lists additional functionality for the core commercial systems.
| Sample of commercial systems required for the management and delivery of cell therapy products. | |
|---|---|
| Systems | Function |
| Cell Orchestration Platform (COP) |
|
| Enterprise and Manufacturing Systems (ERP, MES, LIMS, etc.) |
|
| Quality Management System (QMS) |
|
| Patient Management System |
|
| Courier Management System |
|
| Electronics Clinical Outcome Assessment (eCOA) |
|
When to implement a COP
Up until recently, COP implementation was often delayed until pivotal trials or close to commercialization when growing patient numbers made manual supply chain orchestration increasingly challenging, if not impossible. Over the past few years, companies have recognized the importance implementing a COP early.
The design, configuration, validation and implementation of a COP should not be rushed. This should be planned while patient numbers are still low enough for the supply chain to be managed by alternative means while awaiting implementation. Additionally, established therapies may have long clinical development timelines, giving adequate time for long term supply chain planning. For a growing number of cell therapies, the granting of breakthrough, fast track, orphan and other accelerated review categories shorten the time available to plan for commercialization. As shown in Table 4 the time from treating the first patient in clinical trials to regulatory submission has been as short as under 3 years.
| Timeline of events for Kite Pharma’s axicabtagene ciloleucel for the treatment of aggressive NHL, highlighting the rapid pace from clinical development to commercialization. | |
|---|---|
| Date | Kite Pharma YescartaTM key events |
| December 2014 | Kite Pharma submits investigational new drug application for Phase 1/2 trial of axicabtagene ciloleucel for the treatment of aggressive NHL [13] |
| May 2015 | Kite Pharma announces that the first patient in its Phase 1/2 clinical trial has been treated with axicabtagene ciloleucel for aggressive NHL [14] |
| December 2015 | Kite Pharma receives FDA breakthrough therapy designation for axicabtagene ciloleucel for the treatment of aggressive NHL [15] |
| December 2016 | Kite Pharma initiates submission of U.S. biologics license application for axicabtagene ciloleucel for the treatment of aggressive NHL [16] |
| March 2017 | Kite Pharma completes submission of US biologics license application for axicabtagene ciloleucel as the first CAR-T therapy for the treatment of aggressive NHL [17] |
| October 2017 | Kite Pharma’s Yescarta™ (axicabtagene ciloleucel) becomes first CAR-T therapy approved by the FDA for the treatment of adult patients with aggressive NHL [18] |
A successful COP foundation establishes the pathway and major processes conducted across the supply chain while also pinpointing each step in the process where custody of the product is exchanged and/or chain of identity verification is required. Therefore, the first steps to implementing a COP can be accomplished by establishing clinical site workflows for both collection and infusion and by generating both starting and final drug product labels, courier integrations and milestone reporting within manufacturing environments.
To garner maximum value from the COP, implementation efforts should begin early in clinical development when the number of clinical sites are limited (during phase I/II trials). This early implementation allows for a validated system to be in place to support mid-stage trials, when clinical site numbers begin to expand and manual in-house systems begin to stretch. At this stage of implementation, the combined value of the COP along with the costs saved via introducing the system during early to mid-stage clinical onboarding efforts, instead of onboarding clinical sites without a COP in place, only to have to return to each site for COP testing and training, can immediately justify investment.
Integration strategy for a COP
With the core COP functionality in place, manufacturers should be well positioned to provide an electronic audit file demonstrating chain of identity and chain of custody while also demonstrating capacity management for their biological license application.
This leads us to the second implementation stage (recommended to occur during pivotal trials) that is focused primarily on the integration of commercial systems to maximize efficiencies across the supply cycle. To further increase automation, in order to streamline visibility into the needle-to-needle supply chain, COP integrations may also occur between enterprise, manufacturing and laboratory management systems as each of these systems may contribute valuable information to a COP and stakeholders. A COP can thereby act as a single point of contact for vital information regarding the drug product journey. Furthermore, integration with up and downstream systems such as patient management system and an eCOA system can allow manufacturers to provide additional support and visibility into patient lifecycle stages upstream of starting material collection and downstream of final product infusion. As for the triggering of reimbursement payments, COPs may act as triggering mechanisms to notify the appropriate stakeholder to send invoices or claims. Some therapies will be reimbursed up-front, while other therapies will require milestone or outcome based reimbursements to satisfy the risk-tolerance of payers. The indication, the price of the drug and corresponding pharmacoeconomic data will each play a major factor in formulating the reimbursement model. In the end, COPs can become the overarching platform that tracks and manages the overall patient journey from patient access to final reimbursement.
Commercial view of a personalized cell therapy supply chain
As complex as the supply chain can be, it must result in a seamless and positive experience for the patient and the physicians. The overall patient and physician experience will ultimately contribute towards the differentiation of industry winners and losers. There are many stakeholders involved in the patient lifecycle, including patients, physicians, manufacturers (including contracted case managers), collection centers, couriers and payers. As a result of all the different stakeholders, there is a need for all the players and the required systems to be seamlessly tied together to produce and deliver a drug product. Figure 2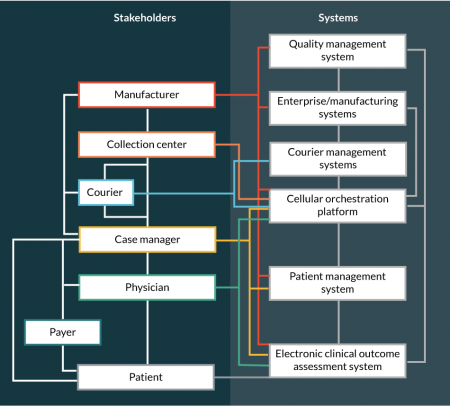
| Table 5: Roles and interactions of major stakeholders in a personalized cell therapy supply chain. | |
|---|---|
| Player | Role and interactions |
| Patient |
|
| Physician |
|
| Collection Center | Collects starting material and associated analytical information Initiates chain of identity and chain of custody audit trail Pack-out and shipment to manufacturing center Interacts with case manager or COP to schedule collection date
|
| Manufacturer (synonymous with cell therapy developer or sponsor during clinical development) |
|
| Courier |
|
| Payer |
|
Managing the complexity of such a supply chain is impossible to perform manually. The success of cell therapies will owe much to whether they can be delivered consistently and reliably, resulting in a positive patient experience.
Patients & Case Management
For the patient and family, being involved in a personalized supply chain can be a daunting and overwhelming experience, especially when the therapy is a last treatment option. Personalized care alleviates some of this burden and hopefully provides a more positive experience for the patient. As such, a single point of contact should be assigned to each patient – a case manager that can work for either the manufacturer or outsourced to a third party patient management support company. For a case manager to seamlessly deliver service to the patient, they will interface with both a COP and a patient management system. They will also require access to reimbursement agents at insurance companies for benefit verification and reimbursement issues.
Following the diagnosis, the patient and/or physician can reach out to a case manager to learn more about the therapy and eligibility. The patient should expect information materials to be provided about the therapy, clinical trial (if still pre-commercial) and options and information for both physicians and collection centers.
Case managers aim to provide the patient with a decision on eligibility as soon as possible. Eligibility will depend on both inclusion criteria for the therapy and insurance coverage for the treatment. For reimbursement support, the case manager will work with the patient, physician and the insurance companies to verify the patient’s insurance and coverage to ensure complete reimbursement for the treatment. In the event insurance coverage cannot be obtained, the case manager should assist the patient in identifying alternative funding options. Considering the financial burden of these therapies will be very high, systems must be in place to assist and provide a more user-friendly experience for the patient and family throughout the entire patient lifecycle.
Once eligibility is received, the patient is scheduled for the collection of the starting material and the subsequent treatments. A case manager will work with the patient to coordinate these appointments, while also providing updates, reminders and requirements for them. Through the utilization of a COP, the case manager will help align all stakeholders, such as the collection centers, manufacturing centers and infusion centers to ensure a seamless transaction. Following the collection of the starting material, the COP can be configured to provide patients visibility into key milestones to provide them comfort that the therapy is on schedule. Most importantly, a COP can ensure that the chain of identity is maintained and provide peace of mind that the patient receives their personalized autologous product and not someone else’s.
Throughout the patient lifecycle the case manager will provide a specialized patient experience, which could include information about the indication and even relevant advocacy groups. They may also play a lead role in care/visit logistics in assisting the patient with booking travel and lodging accommodations for collection centers and infusion centers, as patients may have to travel long distances for therapies.
After the therapy, the patient should expect to have a continued communication with the case manager, as it will be important to ensure the patient adheres to follow-up visits to collect long-term outcome data required by regulators, and also to support payments under outcomes-based reimbursement models.
Physicians
The physician is the stakeholder that triggers the start of the personalized supply chain. However, one of the major challenges for the physician community is coordination between case managers, collection centers and the manufacturing facilities to start the process. COP systems can provide visibility not only into potential collection dates (often driven by manufacturing capacity) but also provides physicians a calendar of all downstream requirements that correspond to a certain start dates, such as preconditioning, infusion date, post-infusion, etc. In addition, the system will be able to provide updates to the physician on the status of manufacturing and drug product release, which will assist in both scheduling of infusion and potential pre-conditioning regimens that may be required. A COP will be able to provide any updates, such as delays, which will allow for the physician to allocate resources appropriately in those instances. At the time of drug delivery, the system can provide specific details on the handling and bedside delivery of the drug product.
Choosing the most effective treatment, while ensuring that it will be financially feasible for the patient, is another challenge for physicians. Through utilization of case managers and associated patient management systems, a physician can receive a quick response regarding patient eligibility, benefit verification and likelihood for treatment reimbursement. This is beneficial to the physician, as internal resources and time are often expended to assist their patients in receiving insurance coverage, and this invested time is not compensated for.
Additionally, the systems need easy-to-use interfaces and portals designed to help consolidate patient forms and reduce paper work redundancies for not only the provider, but the patient. As part of the service, easily accessible information about the therapy and the treatment process should be made available to both the patient and the physician.
Collection Centers
It is a regulatory requirement to track and trace autologous products from collection to infusion [5,6]. To maintain chain of identity, the COP provides collection centers the ability to link the patient’s starting material with the patient’s previously generated identifiers. A COP can capture and produce the required audit trail, by linking the donation identification number (autologous starting material) for each collection to the subject identification number produced by the patient management system. Select COPs can also print labels, with all the required identifiers, that can be affixed to the product bag, or electronically captured within the COP audit trail, prior to delivery to the manufacturing site (Figure 3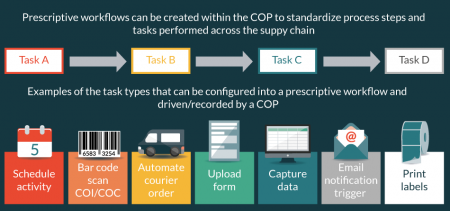
Collecting the starting material can present many challenges to the collection centers as the responsibilities go far beyond the collection and resultant analytical testing. Collection centers are forced to spend a significant amount of manual resources scheduling appointments, couriers and preparing deliveries. A COP can be implemented to help automate scheduling and other logistical tasks. Prior to booking a manufacturing slot, the collection center needs to confirm collection availability to ensure the starting material will be received within the product’s pre-defined stability specification. The system can provide notifications to all stakeholders when an appointment is scheduled/rescheduled/cancelled, allowing for the proper allocation of resources. When scheduling through a COP system, an automatic notification can be sent to the courier with all delivery details at the appropriate point in the collection process. Along with coordinating the courier pickup, scheduling within a COP system can also trigger the courier to have the shipping container available at the time of collection. Finally, the expected time of delivery and other information will be provided to the manufacturing facility via real-time email notifications and dashboard updates. Lastly, all tasks and logistical information will be captured within the audit trail of the COP.
However, in order to set up a COP to fit multiple clinical sites, clinical site diligence should be performed at each of the collection sites. This exercise, which can range from a simple questionnaire to on-site visits, informs the manufacturer about site variations such as site-specific operating procedures and labeling requirements.
As for labeling requirements there is no single approach that works for all manufacturers and clinical sites as standards are not yet in place. However, there are three general approaches to generating starting material labels. The first approach is to leverage clinical site labeling protocols and infrastructure to generate the starting material labels. The challenge with this approach is the inherent site-to-site variability around label requirements and potential inability to electronically establish chain of identity and chain of custody. The second approach is for the manufacturer, or contracted logistics company, to preprint labels and ship to the clinical site. The challenge with this approach is that the labels may be incomplete and require additional information to be added, such as sample weight, volume and other material and collection-specific information. The final approach is to print labels real-time as a COP driven task during the collection process. This approach offers manufacturers the ability to electronically incorporate data generated during the collection process onto the starting material label – eliminating the potential for transcription errors due to hand written entries.
It is important for manufacturers to recognize early that clinical sites may have varying requirements for labels for the outgoing apheresis products. Therefore, if the cell therapy developer is going to provide labels or have a COP print them locally in real-time, it is important to confirm that the site approves the use and design of the labels. Once the manufacturer has a better understanding of the unique operating procedures at the collection sites, a level of standardization can be implemented through training and prescribed software-driven workflows. Industry efforts are underway to standardize cell therapy starting material labels to enable commercialization of the field at large. However, these efforts are nascent and more support is needed from the cell therapy community to galvanize a path forward that provides international harmonization without stifling innovation. Without such effort, clinical sites and manufacturers will be challenged to handle demand as more products enter clinical development and the market. To standardize constructively, input is needed from both commercial manufacturers and the clinical community.
In summary, by eliminating any risk associated with scheduling errors, breaks in chain of identity and chain of custody, labeling and work process variation across clinical sites, the chances of a successful manufacture and patient treatment are increased.
Couriers
Cell therapies are living therapies, and are sensitive to environmental conditions throughout the supply chain. Exposure to abnormal temperatures can significantly impact the viability of a cell therapy product, causing a quality issue even before the start of manufacturing. Timeliness is also a factor, especially when material is shipped fresh and has a short shelf life. Tight coordination of courier activities, in addition to activities performed ahead of courier collection and after delivery, is therefore critical to delivering a safe, effective therapy.
A COP can help to achieve optimal supply chain conditions by defining and implementing product-specific workflows for shipment preparation. This can ensure cellular material is packed appropriately for its required shipping conditions to minimize the risk of temperature deviations in transit.
A COP can also allow sites to automatically order courier collections directly through the system itself. Not only is this convenient for the collection or manufacturing site, but it can also restrict couriers and routes to those pre-selected and approved by the manufacturer. In addition, a COP-driven scheduling system pre-alerts couriers of when collections are required, and automatically updates them in the event of delays, allowing for more efficient planning of transport and ensuring adequate inventory of specialized shipping monitors and components are available when required. COPs can be easily integrated into courier management systems, allowing stakeholders to view the progress of each shipment to their required level of granularity. This can be as simple as key milestone reporting (e.g., collected, in transit, delivered) or, if newer shipment monitors are used, can allow real time tracking of location, temperature, orientation and other factors. Finally, COP-generated alerts can inform sites (manufacturing sites, clinics) of when deliveries are expected. Similarly, alerts or dashboards can be set up in a COP system to inform stakeholders when a delivery was not made as expected, so action can be taken. Overall, the COP eliminates risk for the couriers, collection centers and the manufacturing facilities to ship the wrong product via electronic chain of custody restrictions configured into the system.
Manufacturing Sites
At the highest level, a COP ensures that manufacturing facilities are aware of all incoming products and can be appropriately staffed to initiate manufacturing within the starting material’s shelf life. Secondly, the COP maintains chain of identity and custody upon receipt/dispatch and across the entire manufacturing process while automatically orchestrating events in parallel outside the facility to ensure timely delivery of the final drug product to the patient.
The Importance of a Scheduling System for Manufacturing
Whether manufacturing is occurring at a CMO or an in-house manufacturing facility, awareness of incoming material is critical as the staggering of slot-based manufacturing must be carefully planned as a manufacturing line is pre-reserved for each therapy. Optimal staggering of lot production is not only specific to a particular manufacturing process but also to the clean room configuration, equipment and other resources needed to test and release the drug product.
The criticality of a robust scheduling system becomes further elevated as production volumes increase and clean room suite capacity nears their upward limits. Currently, most cell therapy manufacturing facilities maintain capacity schedules on white boards and spreadsheets that are updated on a weekly basis by staff at the manufacturing facility, yielding available manufacturing slots. This current effort requires significant ongoing communication between case managers, physicians, manufacturing facilities and collection centers to ensure all upstream and downstream resources are aligned for collection and infusion.
Since one of the major functions of a COP is to understand and book manufacturing capacity, it must be easily configurable (and flexible to adapt to process changes) to various manufacturing processes and environments. When configured, the COP system can display manufacturing slot availability to the various stakeholders involved in reserving and booking the capacity. It should be recognized that different system users may require different access rights in addition to different filters. For example, a physician may only be interested in viewing upstream leukapheresis collection options and downstream infusion dates. A case manager on the other hand, may require complete visibility with all events such as collection date and time, courier pick up, manufacturing start, pre-treatments, drug product release, expected arrival at pharmacy and infusion date.
What is most critical to the manufacturer is that once the physician or case manager reserves a manufacturing slot in the COP, the manufacturing facility is notified to confirm the slot. Following confirmation of capacity, the manufacturing facility is consistently updated by the COP system on the arrival status of that inbound material. This simple visibility is incredibly important for a manufacturing facility to ensure allocation of staff across all departments to receive and initiate manufacturing within the appropriate time window.
Maintaining Chain of Identity & Chain of Custody at the Manufacturing Facility
Chain of identity initiates at the clinical site by linking the patient identifier from the patient management system with a donation identification number generated for collection of the starting material. These two identifiers are further linked to the courier waybill and to the manufacturer’s lot number to maintain chain of identity from the collection center, through the manufacturing facility and to the clinical site for infusion.
Depending on the electronic systems in place at the manufacturing facility, the functionality of the COP can be configured to perform foundational actions such as chain of identity, chain of custody and milestone reporting, to broader functionality that additionally includes scheduling, capacity analysis, product segregation, detailed reporting and electronic records (Figure 4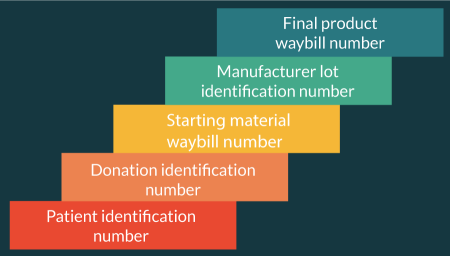
The COP in manufacturing therefore functions first and foremost as the tool to verify chain of identity and custody from the arrival of the starting material to drug product dispatch. Secondly, the system provides manufacturing milestones to all relevant stakeholders in the supply chain. Examples of milestone reporting may include on-time arrival of starting material at a manufacturing facility, start of manufacturing, completion of transduction, successful cell count at a critical decision point, completion of harvest, cryo-storage location, drug product release and pick-up by courier. The milestones can be specific to both the product and the indication, which ultimately drive automated notifications throughout the supply chain. In the end, a COP provides the assurance that the chain of identity and custody is maintained across all departments of the facility from the moment the product arrives to courier dispatch.
Payers
The overall objective for payers is to ensure that the right patient receives the most effective treatment and in the most cost-efficient manner. To execute this, the payers may require long-term outcome data for the drug treatments they are reimbursing. In the cell therapy industry, most companies are still in clinical trials, and even when they commercialize, available data sets are likely to be much smaller than previously seen with more established therapies. Therefore, patient monitoring throughout the entire patient lifecycle, which may last up to 15 years, will be important for payers. This data should be captured via a system, such as eCOA software.
Over time periods that may extend up 15 years, it will be challenging to ensure patients adhere to follow up appointments. A case manager, through the use of a patient management system, can provide notifications to patients and physicians to help ensure this data is collected over this time span. For continued adherence, case managers can also help patients find new physicians, in situations where patients may relocate or enter a new insurance policy. Over time, this adherence support will play an instrumental role in aggregating long-term pharmacovigilance and pharmacoeconomic data for the payer. Lastly, this data will help payers determine if the chosen therapy is indeed the most effective treatment option for the patient and if reimbursement amounts and product eligibility requirements need to be adjusted.
For Kymriah™, the $475,000 cost of the therapy will not be reimbursed to Novartis if the patients do not respond to the drug at the end of the first month [7]. This is an example of an outcome-based reimbursement model that payers are considering. Whereas Kymriah™ only requires one outcome trigger for reimbursement, it is expected that other therapies could require multiple outcomes to trigger payments, potentially over longer durations of the treatment lifecycle.
As we discussed earlier, COPs can integrate standalone systems to provide not only a view of a drug product journey but also a view of the treatment lifecycle. An interface for both the patient and the physician to contribute outcomes data, such as an integration with an eCOA system, would provide a single channel and location for this data to support future outcome-based payments. This will allow for the collection, compilation and analysis of patient outcomes data, which can be provided to payers to both educate and help streamline reimbursement claims.
Future insights
As we look into the crystal ball, what does the next 2–3 years hold for cell therapy supply chains? For starters, the industry will continue to recognize the importance of including the configuration of COPs and adjacent supply chain systems in process development efforts during early and mid-stage stage clinical trials – recognizing that the process goes well beyond the clean room suite. Therefore, process development efforts will not only focus on automation of the manufacturing process or the development of key QC release assays, but will also include early development efforts around the core supply chain stakeholders and the holistic interconnectivity of their corresponding systems. Process development efforts will therefore focus on connecting the systems to automate information flow and the triggering of supply chain events, allowing for seamlessly execution across the various geographical jurisdictions of interest.
Selecting the wrong supply chain vendor or system is comparable to choosing a manufacturing facility that is only US compliant when the long-term goal is to manufacture from a single location for both the USA and the EU. The result of such oversight is redundant efforts, lost opportunity, lost time and lost money. We will continue to see manufacturers shift their logistics mindsets from a short-sighted view, where infrastructure is set up in a piecemeal approach intended to meet the next clinical milestone, to a more strategic approach that considers the holistic commercial strategy and connectivity requirements, both internally and externally. In the end, the piecemeal approach may save a few dollars initially, but more often results in higher cost-of-goods and several disconnected parts that struggle to find synchronicity. Aside from losing time and money in the long run, this ultimately translates into a disjointed experience for the most important stakeholders in the supply chain, the patient and the physician.
This leads to the second area of advancement that we will see in the coming years – the effort by supply chain vendors to develop advanced program interfaces (API) that allow for better connectivity amongst key supply chain stakeholders. The core of these integrations will be with the COP as the systems principle job is to orchestrate. As mentioned throughout this article, one of the major differences between products that require an individual manufacturing campaign for each patient versus more traditional manufacturing campaigns, is the necessity for precise orchestration of stakeholders to deliver a drug, flawlessly, every time.
Not every company will choose the same set of vendors, therefore each supply chain vendor will be pressed to develop APIs that can plug and play with a variety of other systems, allowing for the transmission of key information. Those that recognize the need to be part of a larger interconnected ecosystem will continue to pioneer the growth of this industry, as this is what the industry needs to scale efficiently. However, not all companies will develop APIs with the big picture in mind – some will have better vision and insight than others. A major differentiator between vendors will be the construct of these APIs and how well they understand the holistic data flow and requirements of the collective stakeholders.
Sam Herbert, President of World Courier, delivered this message eloquently in a recent BioInsights interview titled, “Understanding the Critical Impact of Logistics on Scale-up & Commercialization.” In the interview he stated, “Suppliers need to come together to devise an almost off-the-shelf logistics solution that caters to 80% of the supply chain requirements of a cell/gene therapy, requiring only 20% to be customized on a client-by-client basis. By doing this, there’s a real opportunity for suppliers to dramatically contribute to the commercial viability of these products.”
Today, many cross-system integration efforts are already underway between several of the major logistics, enterprise, manufacturing, patient management and reimbursement platforms to provide industry with the essential foundational pieces to create the seamless supply chain. We see an ecosystem of thoughtfully connected companies as a vital step to bring Six Sigma level of quality to the delivery of cell therapies.
Financial & competing interests disclosure
The authors employees of TrakCel. They do not have any financial conflict with the subject matter or materials discussed in the manuscript. This includes, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Direct Technology. (n.d.). Case Study – ECaTS Emergency Call Tracking System- California 9-1-1 Program Office: http://directtechnology.com/right-sidebar-page/business-intelligence/cs-01/
2. Biocompare. (September 24, 2009). Dendreon Reports PROVENGE Regulatory and Commercialization Progress and Future Pipeline Plans at Analyst Event: Website
3. You Tube. (December 9, 2012). The Children’s Hospital of Philadelphia. Emily Whitehead First Child Treated in Trial of T Cell Therapy for Acute Lymphoblastic Leukemia: Website
4. Penn Medicine News. (August 6, 2012). University of Pennsylvania and Novartis Form Alliance to Expand Use of Personalized T Cell Therapy for Cancer Patients: Website
5. Official Journal of the European Union. Directives 2004/23/EC; 2006/17/EC; 2006/86/EC: Website
6. US Food and Drug Administration. (n.d.). CFR – Code of Federal Regulations Title 21, 1271.290: Website
7. Grady, D. (2017 August 30). F.D.A. Approves first gene-altering leukemia treatment, costing $475,000: Website
8. Penn News. (December 7, 2013). Penn Med Team Reports on Study of First 59 Leukemia Patients Who Received Personalized Cellular Therapy: Website
9. Penn Medicine News. (July 7, 2014). University of Pennsylvania’s Personalized Cellular Therapy for Leukemia Receives FDA’s Breakthrough Therapy Designation: Website
10. Novartis. (December 7, 2015). Novartis highlights new CTL019 Phase II data demonstrating 93% complete remission in pediatric patients with r/r ALL: Website
11. Novartis. (June 23, 2017). Novartis pivotal CTL019 6-month follow-up data show durable remission rates in children, young adults with r/r B-cell ALL: Website
12. Novartis. (August 30, 2017). Novartis receives first ever FDA approval for a CAR-T cell therapy, Kymriah(TM) (CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice: Website
13. Kite Pharma. (December 22, 2014). Kite Pharma Submits Investigational New Drug Application for Phase 1/2 Trial of KTE-C19, Anti-CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy, for the Treatment of Refractory Aggressive Non-Hodgkin Lymphoma: Website
14. Kite Pharma. (May 21, 2015). Kite Pharma Announces That the First Patient in Its Phase 1/2 Clinical Trial Has Been Treated With KTE-C19, Anti-CD19 Chimeric Antigen Receptor (CAR) T-Cell Therapy, for Refractory Aggressive Non-Hodgkin’s Lymphoma (NHL): Website
15. Kite Pharma. (December 7, 2015). Kite Pharma Receives FDA Breakthrough Therapy Designation for KTE-C19 for the Treatment of Refractory, Aggressive Non Hodgkin Lymphoma (NHL): Website
16. Kite Pharma. (December 4, 2016). Kite Pharma Initiates Rolling Submission of U.S. Biologics License Application (BLA) for KTE-C19, its Investigational anti-CD19 CAR-T Therapy, for the Treatment of Patients with Relapsed/Refractory Aggressive B-cell Non-Hodgkin Lymphoma (NHL): Website
17. Kite Pharma. (March 31, 2017). Kite Completes Submission of U.S. Biologics License Application (BLA) for Axicabtagene Ciloleucel as the First CAR-T Therapy for the Treatment of Patients With Aggressive Non-Hodgkin Lymphoma (NHL): Website
18. Gilead. (October 18, 2017). Kite’s Yescarta™ (Axicabtagene Ciloleucel) Becomes First CAR T Therapy Approved by the FDA for the Treatment of Adult Patients With Relapsed or Refractory Large B-Cell Lymphoma After Two or More Lines of Systemic Therapy: Website
Affiliations
Martin Lamb1, Robert E Margolin2 & Joseph Vitale3
1 Executive Vice President of Sales and Marketing, TrakCel
2 Vice President of Corporate Development and Strategy, TrakCel
3 Director, Process Engineering, TrakCel
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.