Supply Chain Management in the Delivery of ATMPs for Trials & as Licensed Medicines
Cell Gene Therapy Insights 2017; 3(10), 843-851.
10.18609/cgti.2017.084
Advanced therapy medicinal products (ATMPs) are, without doubt, the most complex medicines being developed and they come with some unique supply chain problems which are often under-estimated or completely misunderstood. The sheer diversity of ATMPs from relatively simple suspensions of somatic cells for immunotherapy or regenerative medicine to complex combination products of cells on medical device scaffolds prevents a comprehensive analysis of supply chain problems but there are very many issues which are universally important or, at least, common to the majority of ATMPs. Similarly, the regulation of cell and tissue therapies differs across the World and critical considerations in one regulatory domain are sometimes irrelevant in others. The term ATMP is unique to the European Union and incorporates all of the cell, gene and tissue therapies which are being developed. However, there are important distinctions across the World where, for example, a combinatorial ATMP consisting of cells applied to a medical device in the EU is not regulated as a medicine in the USA but remains a medical device. This article will focus on the EU ATMP situation but will comment on how the same issues are dealt with in other jurisdictions.
Advanced therapy medicinal products (ATMPs) are, without doubt, the most complex medicines being developed and they come with some unique supply chain problems, which are often under-estimated or completely misunderstood.
The term ‘ATMP’ encompasses a broad range of medicines and includes relatively simple suspensions of somatic cells for immunotherapy or regenerative medicine such as autologous or allogeneic mesenchymal stromal cells, genetically modified cells such as autologous hematopoietic stem cells transduced to carry single gene corrections or T lymphocytes with chimeric antigen receptors and, at their most complex, combination products of cells on medical device scaffolds for tissue engineering. This sheer diversity prevents a comprehensive analysis of supply chain problems but there are very many issues that are universally important or, at least, common to the majority of ATMPs. Similarly, the regulation of cell and tissue therapies differs across the world and critical considerations in one regulatory domain are sometimes irrelevant in others; a good example is the absolute requirement in the European Union (EU) for procurement of human cells as starting materials from licensed and inspected clinical facilities, which is not the case in many other jurisdictions such as the USA. The term ATMP is unique to the EU and incorporates all of the cell, gene and tissue therapies that are being developed. However, there are important distinctions across the world where, for example, a combinatorial ATMP consisting of cells applied to a medical device in the EU is regulated as a medicine whereas it remains a medical device in the USA. This article will focus on the EU ATMP situation but will comment on how the same issues are dealt with in other jurisdictions.
Procurement of starting materials
The unique aspect of most ATMPs is that the starting materials are cells or tissues from patients or volunteer donors so the supply chain begins at a clinical site. For some off-the-shelf, allogeneic products a single volunteer donor or a pool of a small number of donors may be adequate for production of a master cell bank. By contrast, patient-specific products that may be autologous or from an HLA-matched donor require procurement on a product-by-product basis.
In any jurisdiction, the procurement of human tissues or cells requires compliance with good clinical practice but in the EU this requires compliance with the Tissues & Cells Directives (2004/23/EC, 2006/17/EC, 2006/86/EC) [1–3] or the Blood Directive (2004/33/EC) [4] and must occur in an establishment licensed under the relevant directive. This applies to allogeneic and autologous donations. In the case of most ATMPs in clinical trial, the procurement is under the Tissues and Cells Directives and all donors must be screened for statutory infectious disease markers (IDM) on the day of procurement or within 7 days post-donation of the cells/tissues and the testing must be done in an accredited facility. However, the donor cells/tissue will mostly need to begin processing immediately and IDM screening results are not available before the material enters the manufacturing site. It has become common to pre-test donors up to 30 days in advance of the procurement to ensure that the manufacturing site is informed of the infectious risk of the donor material before starting processing. This is in part to protect the scientists and technicians handling the donor material but mostly it is to protect the manufacturing environment from contamination and control the risk of cross-contamination of other products within the facility. The current requirements for donor screening include testing for hepatitis B and C, HIV 1 & 2, HLTV-1 and syphilis with other diseases such as West Nile Virus and hepatitis E being tested on a risk-based approach.
It is important to recognize that a ‘positive’ result for any one of these markers does not necessarily mean exclusion from manufacture. A virally contaminated autologous product can be therapeutically valuable to the individual; e.g., a CAR-T product to treat B-cell lymphoma in an HIV-positive patient. The important consideration is that the manufacturer has a quarantine procedure for potentially infectious products and a manufacturing process that controls the risk to the manufacturing staff and to other products manufactured and stored in the same facility. Consideration should also be taken into account as to how the underlying patient disease could impact the product manufacture as validation is likely to have been performed using healthy donor material.
In order to initiate procurement by clinical sites, the ATMP manufacturer will need to establish a contract with the clinical site and, in the EU, a third party agreement covering roles and responsibilities of the procurement site and the manufacturer to ensure compliance with the procurement licence. These agreements need not be overly demanding but must include:
- A requirement that the procurement site retains a procurement and testing licence for the relevant tissue/cells.
- The site must inform the manufacturer if the licence is suspended or revoked or has conditions placed upon it that impact the supply to the manufacturer.
- The responsibility of the manufacturer to inform the procurer of any adverse event resulting from the supply of tissues/cells and to inform them of any adverse reactions resulting from administration of the tissue/cells after manufacture and supply. These are obligations held by the holder of the procurement licence
- Which party is responsible for transport of the donor materials from the procurement site to the manufacturer.
- The confidentiality and retention of data for the minimum periods proscribed in the relevant legislation under which the procurement is being performed.
Reference to one or more operating procedures that detail how the procurements will be arranged and delivered and the documents linked within a document control system held by the procurement site is essential.
As with any medicinal product, part of the development of any ATMP is the assessment of stability of the starting materials and the optimal temperature range for transport. These data can inform the design of the collection and transport packages and the need for temperature control and monitoring. The stability of the donor material will often define the entire procurement supply chain and a number of models can be envisaged:
- Central manufacture – donor materials must be sufficiently stable for procurement across multiple geographical sites supplying into a global or several regional manufacturing sites (Figure 1)
- Distributed pre-processing with central manufacture – donor materials are time sensitive but can be sent to a national or regional hub for stage 1 processing to provide a more stable starting material (e.g., cryopreserved mononuclear cells) that are then sent to a central manufacturing site. The stage 1 processing center could be a specialized, licensed hospital-based laboratory (Figure 2), or a contract manufacturer or company site (Figure 3)
- Regionally distributed central manufacture – where the manufacturing process can be established in regional company facilities or CMOs, time sensitive donor material can be shipped directly to the manufacturer (Figure 4)
Option 1 is the most secure and requires the least complexity. It can be a comprehensive solution if sufficient manufacturing sites are established to meet the global need. Option 2 can be a cost-effective solution and may be contracted to a manufacturing organization in each region to avoid the need to capital investment. Option 3 appears attractive but the risk to the manufacturer is high since hospital-based cell therapy labs are rarely dedicated to ATMP manufacture and few work to full GMP (cGMP). Outside of the EU many are not even licensed and those that are may have never been inspected for compliance.
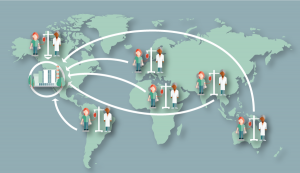
Figure 1. Central manufacture. Donor materials must be sufficiently stable for procurement across multiple geographical sites supplying into a global or several regional manufacturing sites
There is also no single cell cryopreservation method so companies wanting pre-processing and cryopreservation of starting materials by hospital labs will need to import their validated method into each lab and revalidate it within the quality system of the hospital lab and ensure that sufficient staff are trained. Regular audits of the labs will be required during the trial or on-going supply, especially as these labs may have capacity issues that cause a bottleneck for large-scale production.
When the starting material arrives at the manufacturing site the internal supply chain is assured since it will have been validated as part of the process development and validation. However, the supply chain of critical raw materials is as important as the starting material and the process development and validation protocols should include use of raw materials from multiple suppliers wherever possible. We usually validate at least two independent suppliers (one EU and one US) of all critical media, cytokines and sterile disposables and name them in the manufacturing procedure files and regulatory filings. Without this failure of one critical supplier to supply could have huge implications including a halt to all manufacture.
Once the product is manufactured and released for issue from quarantine the supply chain challenges return and are entirely dependent upon the nature of the ATMP. In many cases, the medicinal product is cryopreserved at the final stage of manufacture and quarantined until all quality control and release assays are completed and the head of QC/QA (plus the Qualified Person named on the manufacturing authorisation in the case of EU manufacture) reviews the data and legally releases the product. However, some products cannot be cryopreserved and require immediate release for infusion/implantation within a very short timeframe; sometimes only a few hours. The latter examples require a two-stage release process where in-process QC data are used for the initial release and final release is only possible several weeks later when all of the sterility and other QC data are available. Two-stage release procedures are not uncommon but do require an in depth quality risk assessment and a very controlled process to ensure that the clinical end-users can be contacted as soon as any adverse QC data arise. These are most likely to be microbial contaminants and a process should be set up and tested to ensure that appropriate expert clinical microbiology advice can be given to the clinical end user rapidly. It goes without saying that this must also feed into an adverse event reporting structure to the relevant regulatory agency and a subsequent route cause analysis.
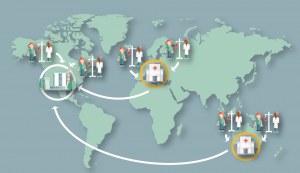
Figure 2. Distributed pre-processing with central manufacture and stage 1 processing occurring at a specialized hospital-based laboratory. Donor materials are time sensitive but can be sent to a national or regional hub for stage 1 processing to provide a more stable starting material (e.g., cryopreserved mononuclear cells) that are then sent to a central manufacturing site. The stage 1 processing center could be a specialized, licensed hospital-based laboratory as shown here or a contract manufacturer or company site (Figure 3).
Transport of ATMPs from the manufacturer to the end user is another aspect of the supply chain that is highly product dependent. All products should be transported under temperature control and with monitoring and a temperature log must be available to the end user and the manufacturer. The nature of the transport container depends upon the temperature of the shipment but it is very common at present for ATMPs to be cryopreserved in vapour phase nitrogen (VPN) and products shipped in dry shippers. This is the current ‘elephant in the room’ for many ATMP developers; especially those with autologous products such as the majority of CAR-T therapies. When the autologous supply chain reaches several thousand doses per year the use of dry shippers for transport becomes a huge challenge. Supply chain experts agree that, at this scale, we will need four dry shippers for every product manufactured; one ready for the current product, one being filled with nitrogen for the next product, one in shipment with the previous product and one empty on its way back from a previous site after infusion of a product. Dry shippers need to be manually filled and must be fully charged at the time of despatch for them to maintain their operating temperature for the full duration of the validated storage period. The short answer is to validate short-term storage at temperatures above -80oC to allow transport on dry ice as has been used for research grade cell transfer for decades. This validation of product stability at -80oC needs to be done during product development so that it is tested during the clinical trial phases. It should be noted that storage of human peripheral blood hematopoietic stem cells at -80oC for many months is a routine practice in some clinical stem cell transplant units so there is adequate precedent for storage at temperatures above -155oC. This problem is potentially less with respect to ‘off-the-shelf’ allogeneic products that could be delivered as multiple doses in a single shipment for local storage. At present this is rarely an option since most hospital pharmacies lack VPN storage capacity and distributed medicines must remain under the control of pharmacy. Once again, validation of storage at -80oC will be valuable in the product development.
Role of pharmacies
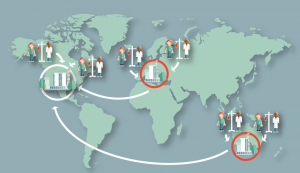
Figure 3. Distributed pre-processing with central manufacture and stage 1 processing occurring at a company site. Donor materials are time sensitive but can be sent to a national or regional hub for stage 1 processing to provide a more stable starting material (e.g., cryopreserved mononuclear cells) that are then sent to a central manufacturing site. The stage 1 processing center could be a specialized, licensed hospital-based laboratory (Figure 2) or a contract manufacturer or company site as shown here.
In many countries, the responsibility for the control of all medicines entering a hospital lies with the local chief pharmacist; this is certainly the case in the UK. Most ATMPs present a challenge to this since they are unlikely to fit into the routine practice; they are either cryopreserved at temperatures below those used in most hospital pharmacies and thus require storage in the delivery dry shipper until delivery to the hospital ward for administration or they must be delivered directly to the clinical end user because of their short shelf life. In some rare cases, it may be necessary to make a final adjustment to the drug product such as thawing and washing and this might need to be contracted to the hospital-based pharmacy. Since the product will have been released by the QP at this stage, the dose cannot be changed so it is important to validate the thawing and washing process during the drug development phase so that the QP can be assured that, if the process is followed, then the dose of the drug product will remain within the released dose specification.
When planning a clinical trial or commercial supply of ATMPs the manufacturer must discuss with the chief pharmacist in the relevant hospitals how they want to control the process of drug delivery to the patient.
The end user
Figure 4. Distributed regional manufacture. Donor materials are too time sensitive for global shipment and it is cost-effective to have multiple regional manufacturing centres.
Finally, once the product arrives at the patient bedside, someone has to administer it to the patient. If the product arrives frozen then the end user must have been trained in how to recover it from the shipping container, how to record the temperature data and how to handle a product safely including the thawing procedure. This training needs to be documented and part of the logistics required in the supply chain is to ensure that a trained member of staff is going to be available at the clinical site when the product arrives and is ready for administration.
To conclude, ATMPs are the ultimate patient-personalized medicines and the complexity of their supply chains is unique; involving hospitals, couriers, pathology laboratories, manufacturers and biopharmaceutical companies. As these move into routine clinical practice the companies developing them and the hospitals administering them require complex partnerships controlled by contracts, confidentiality agreements, training programmes, records and detailed operating procedures, many of which will be specific to individual hospitals. The challenges are considerable and it is never too early to start addressing them.
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. EU Tissue and Cells Directives on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells: Website
2. EU Tissue and Cells Directives on Technical requirements for the donation, procurement and testing of human tissues and cells: Website
3. EU Tissue and Cells Directives on Traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells: Website
4. Technical requirements for blood and blood components: Website
Affiliations
Prof. Mark W Lowdell* and Dr Owen Bain
Centre for Cell, Gene & Tissue Therapeutics, Royal Free London NHS FT
and University College London.
* Author for correspondence: m.lowdell@ucl.ac.uk
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</