Impact of Freeze–Thaw Processes on the Quality of Cells
Cell Gene Therapy Insights 2017; 3(10), 807-813.
10.18609/cgti.2017.069
The unique supply chain involved in the production of cell therapies implies that effective methods of preservation are critical for commercial and clinical application of this form of therapy. Effective methods of preservation enable transportation amongst the different sites (collection, processing and administration), time for completion of safety and quality control testing as well as coordination of the therapy with patient care regimes. The preservation protocol consists of a series of steps (pre-freezing processing, introduction of a cryopreservation solution, freezing, storage, thawing and post-thaw assessment). Errors in each step can reduce post-thaw recovery and cell function. Key scientific concepts are described and resources to help those involved in the development of preservation protocols are provided.
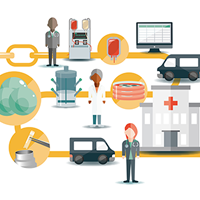
Cell therapy began in the 1970s with bone marrow transplantation for the treatment of blood and immune disorders, and cryopreserved cells have been in use for therapeutic purposes since then [1]. Since that time, the types of cells used therapeutically have continued to grow as does the number of diseases treated with those cells. The supply chain for cell therapies differs from other types of medical therapies (drugs, biologics, medical devices). The complexity of this supply chain is shown schematically in Figure 
The use of cells therapeutically also brings with it the requirement that the cells be tested prior to administration to meet safety and quality control requirements, which may take hours or even weeks to complete. The ability to preserve cells enables completion of safety and quality control testing prior to administration of the cells. The recipients of these cells are sick patients. The ability to preserve cells helps medical professionals coordinate availability of the therapy with patient care regimes. Finally, the ability to preserve cells enables development of a manufacturing paradigm for cell therapies where cells are produced and stored until use. This capability reduces cost for production by enabling management of inventory, equipment and staffing for cell processing facilities.
Why do we freeze cells to preserve them?
The goal of the cryopreservation process is to retain the critical biological properties of the cells post-thaw. On a molecular level, freezing enables us to achieve the goal of stabilizing the cells by reducing the mobility of water molecules by transforming the liquid water into ice from the sample; thereby inhibiting the ability of water molecules to participate in degradation of the sample (see [2] for review). All cells contain degradative enzymes (DNAses, proteases etc.) that act to degrade the cell. The preservation of cells at low temperatures is effective because the activity of those enzymes is a function of temperature. As the temperature is reduced, the activity of the enzymes decreases and there is a threshold at which the protein is no longer active and therefore cannot participate in degradation of the cell.
The process is the product
Unlike a lot of other bioprocesses, the properties of the end product (viability, recovery and function of the cells post-thaw) represent the cumulative effect of all of the processing steps and all of the reagents used in the process (Figure 2
Processing of cells for cryopreservation can be divided into a series of steps: pre-freeze processing, introduction of a cryopreservation solution, freezing, storage and thawing and post-thaw assessment. Poor practices or common errors can result in damage and cell losses. A brief discussion of each of these steps and the scientific basis for the step will be described.
Pre-freeze processing
Cells are collected from a donor and the manner by which this process is performed may influence post-thaw recovery. Even if the collection process is not controlled or standardized, specific pre-analytical factors should be noted in the production record [3]. For example, cells collected using apheresis should also include information on:
- Anesthesia used
- Collection technique (location, etc.)
- Anticoagulant
- Volume collected
- Number of nucleated cells harvested
- Time and temperature between collection and processing
- Centrifugation (duration and g-force used)
- Bag and anticoagulant
- Centrifugation
- Filtration
- Separation of mononuclear cells
The cells collected are commonly processed to produce the final therapeutic product. Common processing steps include (but are not limited to):
- Culture
- Selection of subpopulations
- Activation
- Isolation from a tissue
Hypothermic storage
Each of these steps can stress the cells and cell losses during processing should be monitored and qualified. For example, poor culture conditions (deficits in nutrients/oxygen, over passaging) can influence the ability of the cells to survive the stresses of freezing and thawing. Sub-lethal stresses can influence post-thaw recovery and cell health, not just cell membrane integrity, should be monitored during the pre-freeze processing.
Introduction of the cryopreservation solution
Cells are frozen in specialized solutions designed to help them survive the stresses of freezing and thawing. Additives that help the cells survive freezing and thawing are more commonly called cryoprotective agents. The two most commonly used cryoprotective agents are dimethylsufoxide (DMSO) and glycerol. As cells cannot be filtered or purified, the components of a cryopreservation solution must be pure, potent and safe. Studies have demonstrated that residual contaminants in certain cryoprotective agents can influence post-thaw recovery [4].
Cryopreservation solutions are typically hypertonic. For example, a 10% vol/vol DMSO solution is ~1400 mOSM, and simply introducing the solution or exposing cells to the solution can result in cell loss/death resulting from osmotic stresses. When cells are placed in a cryopreservation solution, the initial response of the cells will be to dehydrate in order to reduce the chemical potential difference between the intracellular and extracellular solution rapidly followed by a slow increase in cell volume resulting from penetration of the cryoprotective into the cell. If these volumetric excursions are large enough, the cells will lyse upon introduction of the cryopreservation solution [5]. Similar problems are faced upon removal as most cells need to be washed or diluted prior to use post-thaw. When the cells are then transferred from a high osmolarity solution to a low osmolarity solution during washing, water rushes in rapidly resulting in a rapid growth in cell volume followed by slower removal of the small molecular weight cryoprotective agent. Cells are more sensitive to osmotic stresses post-thaw and therefore cell losses from removal can be significant. These cell losses are independent of the freezing process. Certain cryoprotective agents such as DMSO are toxic to the cells and result in cell losses with time of exposure to the solution [6–9]. DMSO has been shown to alter the cytoskeleton, shift metabolism of cells and change membrane fluidity [6] and these effects can result in loss in cell viability with time. Best practices involve developing and optimizing a process for introduction and removal of a solution that minimizes cell losses [8].
Freezing
The freezing process takes a cell from a physiological temperature (~37°C) to a cryogenic temperature (-196°C). The rate at which that is done (i.e., the cooling rate) is a key factor in cell survival [10]. Controlled-rate freezers or passive freezing devices (e.g., Mr. Frosty or CoolCell) are used to control the rate at which cells cool. Protocols for controlled cooling of samples can be complex and involve multiple processes. Each process (described most often as a segment) can be rationally designed and optimized. When a protocol specifies a cooling rate of 1°C/min, that is the cooling rate observed at high subzero temperatures (~0 to -40°C) where response of the cell to the freezing environment is greatest.
In addition to cooling rate, the temperature at which ice forms in the extracellular solution can influence the post-thaw viability of cells frozen in a given solution at a given cooling rate. Certain cell types are more sensitive to changes in the temperature at which ice forms in the extracellular solution (e.g., nucleation temperature) than others. Cells that are sensitive require controlled cooling rate freezing.
Storage
After completion of the freezing process, cells are typically stored in a repository until use. As described previously, the freezing process involves reducing the mobility of water and the activity of degradative enzymes. Storage of samples should take place at temperatures below which the sample is stable. For cells, best practice require that cells be stored at temperatures below -150°C [11]. The stability of cells in storage is most strongly influenced by two factors: transient warming [12,13] and background ionizing radiation [14,15]. Proper training and access protocols can help reduce transient warming of samples during retrieval of samples from a repository. The shelf life of a cell therapy product during storage is a critical issue. Future work in the area is needed to develop standard methods of determining shelf life and stability in storage. It is common for samples to be used at a different location and the sample may be shipped to that location. Maintaining a low temperature during shipping is critical to maintaining the quality of the sample during shipping. Dry shippers are most often used for transportation of cryopreserved cells.
Thawing/warming
The range of temperatures traversed during freezing is also traversed during thawing (but in reverse). As with cooling, the manner in which this process is performed is important to post-thaw recovery. The thawing rate must be more than one order of magnitude greater than the cooling rate for most conventionally cryopreserved cells [16]. Typically, thawing rates of > 60°C/min are desired. Characterizing the thawing process is important in validating this particular step in a protocol. It is possible to estimate the warming rate using a stopwatch. The average warming rate is the change in temperature (melting temperature of the solution minus the storage temperature) divided by the time to thaw. Adding a thermocouple to monitor the temperature during thawing can improve accuracy of estimating thawing rates. Controlled thawing devices are being commercialized and should improve the consistency and control of the thawing process. Certain cell therapies are infused directly post-thaw but others may be washed to remove the cryoprotective agents or diluted.
Post-thaw assessment
Determining the outcome of a cryopreservation protocol requires use of effective and meaningful methods of assessing the quality of the sample post-thaw. Poor methods of post-thaw assessment are common. Three specific practices can be used that reduces the common errors or measurement biases observed using common methods of post-thaw assessment
- A thawed product will contain three different populations of cells: (1) cells that have survived and are intact; (2) cells that are intact but not viable; and (3) cells that have lysed. Measuring post-thaw recovery (the number of viable cells post-thaw divided by the number of viable cells pre-freeze) eliminates the measurement bias associated with counting only intact cells.
- A fraction of the intact cells present in the sample will undergo post-thaw apoptosis [17]. Post-thaw assessment should be performed at a consistent time post-thaw in order to reduce variations in the measures resulting from post-thaw cell losses.
- In particular for cell therapies, post-thaw function is critical. Use of multiple measures of post-thaw assessment (in particular during methods development) is critical to the development of a cryopreservation protocol that results in a high number of functional cells post-thaw.
Reproducibility
Effective use of cell therapies in a clinical context requires development of a reproducible process. As cryopreservation is a part of the overall processing of the cells, it is critical that the preservation process also be reproducible. Specific factors have been identified that resulted in poor reproducibility of cryopreservation protocols [18]. These factors include:
- High levels of cell stress prior to cryopreservation
- Improper formulation of freezing or thawing media
- Use of expired freezing media
- Improper freezing or storage protocols
- Incorrect thawing of cells
These factors link directly to the protocol steps described previously (Figure 2). Additional resources for those interested in developing a complete cryopreservation protocol for cell therapies or improving an existing protocol can be found in [19].
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Appelbaum FR, Herzig GP, Ziegler JL et al. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood 1978; 52: 85–95.
2. Hubel A, Spindler R, Skubitz AP. Storage of human biospecimens: selection of the optimal storage temperature. Biopreserv. Biobank. 2014; 12: 165–75.
CrossRef
3. Betsou F, Lehmann S, Ashton G et al. Standard preanalytical coding for biospecimens: defining the sample PREanalytical code. Cancer Epidemiol. Biomarkers Prev. 2010; 19: 1004–11.
CrossRef
4. Sputtek A, Sputtek R. Cryopreservation in Transfusion Medicine and Hematology. In: Life in the Frozen State, Fuller BJ, Lane N, Benson E (Eds). CRC Press, Boca Raton, FL, USA, 483–504 (2004).
CrossRef
5. Levin RL, Cravalho EG, Huggins CE. A membrane model describing the effect of temperature on the water conductivity of erythrocyte membranes at subzero temperatures. Cryobiology 1976; 13: 415–29.
CrossRef
6. Fahy GM. The relevance of cryoprotectant “toxicity” to cryobiology. Cryobiology 1986; 23: 1–13.
CrossRef
7. Fahy GM. Cryoprotectant toxicity neutralization. Cryobiology 2010; 60, S45–53.
CrossRef
8. Fahy GM, Lilley TH, Linsdell H, Douglas MS, Meryman HT. Cryoprotectant toxicity and cryoprotectant toxicity reduction: in search of molecular mechanisms. Cryobiology 1990; 27: 247–68.
CrossRef
9. Fahy GM, Wowk B. Principles of cryopreservation by vitrification. Methods Mol. Biol. 2015; 1257: 21–82.
CrossRef
10. Leibo SP, Mazur P. The role of cooling rates in low-temperature preservation. Cryobiology 1971; 8, 447–52.
CrossRef
11. USP. US Pharmacopeia-National Formulary Standards for Biologics. In: Cryopreservation of Cells, Volume 1044. US Pharmacopeia, Washington DC, USA, 1–31 (2014).
12. Cosentino M, Corwin W, Baust JG et al. Preliminary Report: Evaluation of Storage Conditions and Cryococktails during Peripheral Blood Mononuclear Cell Cryopreservation. Cell Preserv. Tech. 2007; 4: 189–204.
CrossRef
13. Yang J, Diaz N, Adelsberger J et al. The effects of storage temperature on PBMC gene expression. BMC Immunol. 2016; 17: 6.
CrossRef
14. Cugia G, Centis F, Del Zotto G et al. High survival of frozen cells irradiated with gamma radiation. Radiat. Prot. Dosimetry 2011; 143: 237–40.
CrossRef
15. Ozawa K, Miura Y, Suda T, Motoyoshi K, Takaku F. Radiation sensitivity of leukemic progenitor cells in acute nonlymphocytic leukemia. Cancer Res. 1983; 43: 2339–41.
16. Mazur P. Principles of Cryobiology. In: Life in the Frozen State. Fuller BJ, Lane N, Benson E (Eds). CRC Press, Boca Raton, FL, USA, 3–66 (2004).
CrossRef
17. Stroh C, Cassens U, Samraj AK, Sibrowski W, Schulze-Osthoff K, Los M. The role of caspases in cryoinjury: caspase inhibition strongly improves the recovery of cryopreserved hematopoietic and other cells. FASEB J. 2002; 16, 1651–3.
CrossRef
18. Freedman LP, Gibson MC, Neve RM. Changing the Culture of Cell Culture: Applying Best Practices and Authentication to Ensure Scientific Reproducibility. Biopharm. Int. 2015; 28, 14–20.
19. Hubel A. Preservation Of Cells: A Practical Manual. Wiley, NY, USA (2018).
CrossRef
Affiliations
Allison Hubel1,2
1 Biopreservation Core Resource (www.biocor.umn.edu), University of Minnesota, USA.
2 Mechanical Engineering Department, University of Minnesota, USA.
hubel001@umn.edu
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License</