The Importance of Understanding & Designing Cellular Therapy Supply Chains
Cell Gene Therapy Insights 2017; 3(10), 873-889.
10.18609/cgti.2017.087
The objective of this article is to propose a revised conceptual framework for the analysis of the supply chains for cellular therapies that considers first the impact of supply and demand uncertainty on the supply chain pathway, and second the impact of complexity on the physical supply network configuration. We suggest a revised cellular therapeutics supply chain taxonomy that emphasizes the interdependence between product characteristics, supply chain pathway and network configuration, which we hope will facilitate structured thinking around the design of optimal supply chain strategies for different products.
The potential for cellular therapies to be transformative to the treatment of degenerative and neoplastic diseases is undoubted. They are, however, complex products with a high degree of variability in starting materials, manufacturing processes and clinical effects. Great emphasis has been placed (rightly) on the challenges in manufacturing such products, but equally weight needs to be given to understanding the nature and management of the supply chain. Product development must take an integrated end-to-end supply chain perspective if this new generation of therapies is to realize widespread adoption by healthcare systems.
This article argues that the development of cellular therapeutics must consider the complexities associated with each of the key supply chain processes including incoming, manufacturing and outgoing pathways. Cellular therapies lie at a nexus between the supply chain(s) that underpin pharmaceutical products and those that underpin substances of human origin (SoHO) used for human therapy – blood components, cells, tissues and organs. Consistent with this perspective, the quality management system (or concatenated systems) and regulatory compliance must encompass the full provenance of the cellular therapeutic. Finally these therapies need to be used with clinical precision in terms of an understanding of the etiology and pathogenesis of seemingly homogeneous disease ‘entities’ and the place of the intervention in increasingly complex patient care pathways. The product, the procedure and the post-procedural care (e.g., immunosuppression or rehabilitative care) may all be critical to the therapeutic success of the intervention. Recognising that cellular therapies are diverse, Teng et al proposed a taxonomy that differentiates products on the basis of degree of supply and demand uncertainty [1]. We believe this is a good basis for defining optimal supply chain pathways but needs to feed into the broader consideration of complexity in each of the key elements of the supply chain. Using this emergent taxonomy we then develop a framework and logic to support the selection of an appropriate supply network configuration. Finally, the characteristics of the product, the supply chain pathway and physical network configuration need to be considered together as the main elements of an overarching supply chain strategy.
A generic supply chain for SoHO
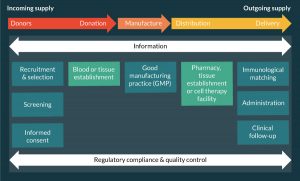
In the order of 100 million blood components (red cells, platelets and plasma) transfusions, tissue (bone, tendon, heart valve, skin) transplants, hematopoietic stem cell (HSC) transplants and sold organ (kidney, liver, pancreas, heart, lung and small bowel) transplants are carried out per annum worldwide for a wide variety of clinical conditions. These products share a similar generic supply chain (Figure 1) whilst varying widely in the detail of their supply chains dependent on the nature of the product.
Thus SoHO intended for human therapeutic use require a donor or donors. Those individuals may be autologous (the cells or tissue is being procured for their own use) or allogeneic (the cells or tissue is being procured for the use of others), and the latter may be living or deceased. They are usually selected on the basis of immunological phenotype (e.g., blood group antigens, human leukocyte antigens), medical history and low risk of infectious disease and will undergo microbiological screening. The cells, tissues or organs need to be procured in such a way as to protect the health of both the donor (if he/she is living) and the potential recipient(s). There is a manufacturing process of variable complexity and the product may be stored prior to distribution to the clinical environment and administration to the patient. Whilst starting materials are developed into products from left to right across the supply chain, information, quality assurance and regulatory compliance are bidirectional and comprehensive, i.e., cover the whole scope of the supply chain.
Thus the generic SoHO supply chain differs from that of pharmaceuticals in respect of the need for a human donor, the complex and variable nature of the product and the need for precision in matching the product to the recipient and his/her clinical condition. In addition the relationship between donor and recipient may be 1:1 or 1:n where n = a fairly small number of patients, for example, up to 3 in the case of a blood donation. In this context, increased product quantity needs to be achieved through out-scale rather than up-scale. Again this differs from a typical pharmaceutical supply chain where production of a relatively standard and stable product can be up-scaled to be administered to very large (N) numbers of patients.
In reality, SoHO vary significantly in the nature of their supply chains dependent on the nature and degree of patient specificity of the product. For example, some products are relatively labile such as platelet concentrates (which require storage at +22°C and have a 7 day shelf life with constant agitation) and solid organs (which are transported on ice with an 18–24 hour maximum cold ischemia time). Others, such as plasma and HSC can be cryopreserved and maintained for several years, but need controlled and precise thawing in a clinical environment prior to administration. Some products are fairly generic in terms of their specificity for individual patients, for example group O RhD-negative red cell concentrates can be safely administered to around 98% of patients. Other patients, such as those with multiple red cell alloantibodies, require individual donors of known red cell antigen phenotype to be recruited. Similarly bone can be administered to any patient (without immunological matching) whilst autologous or allogeneic HSC are highly specific to an individual and as such require precise matching. These variable requirements drive different supply chain strategies.
The incoming supply chain for cellular therapeutics
Cellular therapeutics are derived from human blood, cells or tissues as starting materials and therefore are subject to a similar incoming supply chain path to other SoHO regulated under blood or tissues regulations. Similar considerations apply to the recruitment, selection and screening of the donor, the nature of the donation procedure, any requirement for primary processing and control of the environment in which the starting material is distributed to the manufacturing facility.
The manufacture of cellular therapeutics
The complexity and potential scalability of the differing manufacturing processes for cellular therapeutics are diverse:
Minimally manipulated cellular therapeutics
The most relevant paradigm for which is the established field of HSC transplantation, where mononuclear cells procured from the patient (autologous) or a donor (allogeneic) bone marrow, mobilized peripheral blood or umbilical cord blood are transplanted fresh or cryopreserved and thawed just prior to transplantation. Other products in the category include: preparation of enriched cell populations on the basis of immunophenotypic markers such as CD34 or CD133, either for hematopoietic transplantation (homologous use) [2] or for other clinical indications, for example to improve post-myocardial infarction perfusion (heterologous use) [3]; and islet cell isolation from pancreata for transplantation via hepatic portal vein infusion for unstable diabetes mellitus with hypoglycaemic unawareness [4]. In Europe, this category of cellular therapeutics is normally regulated under tissues regulations, apart from those intended for heterologous use.
Somatic cellular therapeutics
Where cells are cultured for a limited period of time in vitro (often for a matter of days, up to a week or two). Examples include autologous macrophages for the treatment of liver cirrhosis [5] and autologous tumor infiltrating lymphocytes (e.g., [6]). Some somatic cells are cultured for a more prolonged period of time in vitro (typically up to 8–12 weeks) prior to transplantation potentially into larger numbers of (allogeneic) recipients. Examples include anti-viral cytotoxic T lymphocytes (CTLs) [7] and mesenchymal stromal cells (MSCs) [8] for the treatment of a variety of autoimmune diseases or to ameliorate graft-versus-host disease.
Genetically modified cellular therapeutics
Some cell products may be genetically modified, for example by transduction of T cells with modified T-cell receptors or chimeric antigen receptors (CAR) [9] in order to target cancer-associated antigens, and genetically modified HSCs [10]. Whereas, over previous years, genetic replacement of abnormal genes has been carried out mainly using plasmids or viral vectors, the development of a new generation of gene editing tools (such as CRISPR-Cas9) is beginning to open up gene correction as a potential therapeutic pathway [11].
Reprogramming
Pluripotent stem cell lines can be derived either from in vitro blastocysts – human embryonic stem cells (hESCs) or reprogramming of adult cells using transcription factors – induced pluripotent stem cells (iPSCs). Such cell lines can proliferate indefinitely in culture and differentiate into most cell types. They therefore open the possibility of generating a variety of different cellular therapeutics for an individual on an autologous or allogeneic basis or of a single allogeneic donor contributing multiple products to a very large number of recipients, potentially over an extended period of time. Examples include hESC-derived retinal pigment epithelium cells for age-related macular degeneration [12] and iPSC-derived red cells or platelets [13].
Tissue engineered products
Cells exist mainly as part of complex 3D tissue structures in vivo rather than as homogeneous cell suspensions. Both somatic cells and cells produced from reprogrammed tissues may be used to populate natural or synthetic extracellular matrices. Decellularised dermal, tracheal and esophageal tissues (e.g., [14]) have been repopulated with autologous or allogeneic mesenchymal and epithelial cells as a potential approach to the correction of structural defects. In addition, there is emerging evidence that mixtures of cells can self-organize into structured tissues (organoids) [15].
In terms of scalability of manufacture, similar to the situation with SoHO, a spectrum of cellular therapies exists in terms of the relationship between donor and recipient(s): some products such as corneal epithelial stem cells may be autologous (by definition 1:1) or allogeneic 1:1 or 1:n (where n = a few recipients) and therefore require small unit out-scaled manufacturing [16]. Others, such as MSC- or PSC-derived products may be amenable to larger scale allogeneic 1:N (where N = many recipients) manufacturing.
Cellular therapy products in the latter four categories are regulated as medicinal products in most jurisdictions and as such require a Marketing Authorization (or similar) in the same way as other pharmaceutical agents.
The outgoing supply chain for cellular therapeutics
Typically, the complexity and scalability of manufacture have been considered to be the major factors determining whether the supply chain for cellular therapies is likely to require decentralized or centralized manufacturing. However, this binary division is an oversimplification because the variable stability of products and the need for personalized administration adds complexity to the outgoing supply chain path.
Some products are labile and have to be delivered within a limited shelf life and in a tightly controlled environment (corneal epithelial stem cells are a good example), whilst others may be able to be cryopreserved and thawed in the healthcare environment (MSCs are a typical example). Somewhere between these lie products that may be able to be transported frozen but require some form of secondary processing or manufacture which renders them into a more labile product prior to administration. This is typically a part of the supply chain that manufacturers struggle with since the ‘pharmaceutical’ model is predicated upon a stable product that can be stored at ambient temperature with relatively minimal monitoring and generic applicability to a patient population.
Precision matching between the cellular therapeutic product & the patient
The fact that cellular therapies express similar tissue antigens to other SoHO means that a degree of immunological matching between donor and recipient(s) is likely to be required. Hence ABO carbohydrate antigens are expressed by all cells and major incompatibility (e.g., administering a group O product to a group A individual) will lead to hyper-acute rejection. Incompatibility at major HLA-loci, particularly Class I is also likely to lead to acute or chronic rejection, particularly in patients with pre-existing anti-HLA alloantibodies. Minor histocompatibility antigens and polymorphisms in natural killer cell receptors may also contribute to immune incompatibility between the cellular therapy product and the recipient. Thus some degree of immune suppression is likely to be required to reduce the risk of rejection of allogeneic cellular therapies; however, the better the immunological match the more this is likely to be able to be mitigated. Given the cost of immunosuppression in financial and health terms (immunosuppression increases the risk of infection, neoplasia and a variety of tissue toxicities), maximizing the degree of tissue matching is desirable for most products in both individual and societal terms. As an example, it has been suggested that clinical-grade iPSC should be derived from a set of donors who are group O and homozygous at the major class I and II loci to provide a set of differentiated products which are the best match with the widest range of recipients [17].
Many cellular therapies will also need to be matched in respect of detailed diagnostic criteria in the recipient patient population. It has become clear over recent years that disease entities or processes previously considered homogeneous are often highly diverse when analyzed at a molecular level. For example, CTL containing genetically engineered TCR may be specific to patients of restricted HLA type and tumors expressing specific antigens [18]. Similarly the success of a stem cell administration may depend upon whether the primary problem is stem cell deficiency per se or deficiency within the stem cell niche or microenvironment. In this and related fields, we are beginning to see the development of companion diagnostics that guide the application of these precision therapies to highly specified disease subgroups. It is likely that most cellular therapeutics will need to be carefully tailored to disease and patient characteristics and a ‘one size fits all’ approach to the manufacture and administration of these products is unlikely to be successful.
Clinical administration
Finally, consideration needs to be given to the complexity of administration to the patient. Typically pharmaceuticals are stable products that can be administered systemically to relatively large populations of patients. Pharmacies are constituted to deliver licensed Medicinal Products (i.e., post-clinical trial) but most will not currently have the facilities require to manage labile cellular products or those that require secondary manufacture. Some cellular therapies will also be capable of straightforward intravenous administration where they are capable of homing or exerting their therapeutic effect from a non-specific location. Many others will, however, require site-specific administration either through surgery or interventional radiology. Moreover, long-term specialized follow up might be required for post-procedural clinical management including adverse reactions or side effects of the product. Scheduling of interventional and monitoring procedures in a busy healthcare environment is notoriously vulnerable to uncontrollable externalities such as the health of the patient and the last minute re-purposing of facilities to urgent cases. Thus it is likely that clinical services will need to be realigned to a certain extent to facilitate the delivery of this unusual class of therapeutic agents.
Differentiated supply chain design
To ensure the commercial and clinical implementation of new cellular therapeutics, developers must recognize that success is not solely based on offering innovative and customized products but also on product affordability and customer service, attributes which are dependent on effective and efficient supply chain capabilities. It is well understood that products with different characteristics e.g., volume, value, shelf-life or substitutability [19–21] serving diverse market segments in terms of uncertainty of, or instability in, demand and geographic location [22] require different supply chain strategies [19,23]. As researchers have developed this differentiated approach it has become evident that the product development process and the likely supply chain design need to be aligned [24] to maximize the chances of success. Information exchange between product developers and supply chain managers is essential and can be achieved through the integration of supply chain representatives in the product development process [25].
Several frameworks have been proposed [19,23,26] to guide the design of supply chains to suite different categories of products, however Hilletofth [24,27] argues that these models are too simplistic as they constrain the choice of strategy, i.e., lean, agile or hybrid, rather than ensuring the optimal combination of sourcing, manufacturing and distribution strategies. These models also suggest just two [19,26] or four [23] product segments that fail to reflect the true diversity in the products offered and the complexity of the environment.
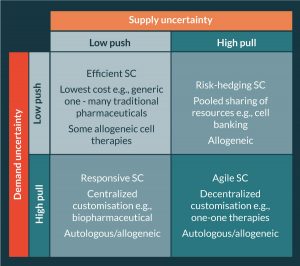
Teng et al. [1] propose a cell source taxonomy for categorizing cellular therapeutics. The taxonomy distinguishes between one-to-one (autologous or allogeneic) or one-to-many (allogeneic) therapies before segmenting by demand volume (high or low) and finally delivery mechanism (procedural or product). The authors then reference the work of Fisher [19] and Lee [28] and present a supply chain uncertainty framework (Figure 2), commonly applied in the retail sector, to identify supply chain pathways for different types of therapies and argue that the classic uncertainty framework applies equally to pharmaceutical, biopharmaceutical and cell therapy supply chains.
Our critique of Teng’s framework as applied to cellular therapeutics is that whilst it identifies supply and demand uncertainty as a driver of the supply chain pathway for different products and emphasizes the importance of identifying decoupling points, it conflates this with the source of the starting material (autologous or allogeneic) and choices around geographical configuration of the supply chain (i.e., decisions around the extent to which its necessary to centralize or decentralize different elements of the supply network), which are contingent upon the relative complexity of procurement, manufacture and distribution. When selecting an overarching supply chain strategy for a particular therapy we must therefore consider first the impact of supply and demand uncertainty on the supply chain pathway and second the impact of complexity on the physical supply network configuration.
Accounting for uncertainty in cellular therapy supply chain pathways
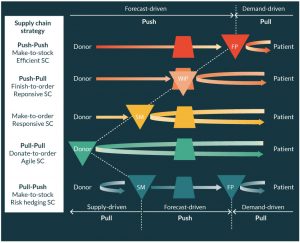
High volume ‘functional’ products typically exhibit relatively stable and therefore predictable demand (i.e., low demand uncertainty) and hence production and distribution decisions can be made ahead of demand based on forecasts (make-to-stock) without significant risk of obsolescence or poor availability. In these push–push (efficient) supply chains, the emphasis is on taking advantage of economies of scale (EoS) in production and transport so as to minimize the total unit cost. The product is pushed through the supply chain to the customer interface where it is held in stock ready to satisfy customer demand ‘off the shelf’. Although manufacturing may be centralized to take full advantage of EoS in manufacturing, the final product stock-holding is dispersed in a decentralized network. Many pharmaceutical products adopt this supply chain strategy.
When demand is uncertain (typically low volume ‘innovative’ products) and difficult to forecast a push–pull (responsive) or a pull–pull (agile) strategy becomes more effective. In a push–pull supply chain, starting and raw materials are usually procured to meet a forecast and held in stock at the decoupling point (where push meets pull); when a specific customer order (or patient need) is identified then the material is ‘pulled’ from the decoupling point through production and delivered to satisfy the order (make-to-order). In a pull–pull supply chain, nothing is procured or processed until a specific customer need arises, in the case of some SoHO supply chains the donor may, in effect, be the material decoupling point.
Finally the very unusual pull–push supply chain is seen in markets where supply may be sporadic and hence the high supply uncertainty favors a pull strategy, i.e., when there is available supply then the starting material is pulled into the supply chain and held as stock until it is needed to satisfy demand. To help illustrate these different concepts and supply chain pathways a generic representation for SoHO is provided in Figure 3.
Accounting for complexity in cellular therapy supply network configuration
Whilst supply and demand uncertainty is one key driver of the choice of supply chain pathway, in the field of cellular therapies other key considerations also come into play such as the stability of the starting materials, intermediary and final products, the extent to which a product is generic or specific to a particular patient, and the degree of clinical urgency with which the product might be required set against the time to manufacture and deliver. Different elements of the supply chain (i.e., donation, manufacturing and distribution) may also differ significantly in respect of the level of process complexity. As a process becomes more complex with more steps and more interaction between those steps, it utilizes more resources and requires more specialist management. The incoming supply chain may be complex in respect of the need to obtain starting material from highly specified (e.g., HLA-matched allogeneic) donors, may require specialist donation procedures or may require primary processing near to the point of donation. Distribution to the manufacturing center may be time-bounded and/or require a carefully controlled environment if the starting material is labile. Manufacturing may vary significantly (and independently) in both complexity and scale. The outgoing supply arm may be complex in respect of the lability and shelf life of the final manufactured product, the need for tertiary processing, the extent of matching required between the product and the donor or the nature of the clinical procedure required for administration. The need for specialist logistics (storage and transportation) and the time to execute process steps (i.e., lead time) will contribute to the complexity of incoming and outgoing supply. For example, for a time-sensitive labile product the longer the in-bound and out-bound logistics lead time the greater the risk of product deterioration or shelf life expiry. Lead time is affected by the physical proximity of procurement sites, manufacturing centers and hospitals to each other and hence must be considered in the evaluation of supply chain complexity.
Thus, rather than assessing the uncertainty and complexity of a supply chain as a single unit of analysis as depicted in Figure 2, when designing a cellular therapeutic supply chain strategy we advocate considering supply and demand uncertainty as a first step in determining an optimal supply chain pathway (Figure 3 and then overlaying this with consideration of the complexity and constraints within and between each of the three main components of the supply chain (incoming, manufacturing and outgoing) in order to determine the optimal supply network configuration (Figures 4–7).
Multi-dimensional variables of this kind suggest higher-order complexity across cellular therapeutic supply networks (i.e., complexity not just within different supply chain echelons but between echelons) and require a more subtle taxonomy and a flexible combinatorial approach to overall supply chain strategy.
Towards a new taxonomy of cellular therapeutics
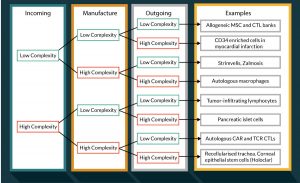
Given the above analysis a new taxonomy of cellular therapeutics can be proposed based on the level of complexity in donation, manufacture and distribution (Figure 4).
Whilst this classification can be criticized in respect that each of the three echelons contains multiple elements which may differ in complexity and that that complexity is a continuum rather than binary in nature, it is clear that there is a need for a wide range of different cellular therapeutics supply chains and that no single model is likely to afford the infrastructure to support widespread clinical trialing and adoption of this class of therapies.
Impact of complexity cost trade-off on network configuration
Much of the discussion within the community to date has been focused on the question of up-scaling or out-scaling cellular therapy production to meet future demand. Due to the nature and complexity of a cell therapy supply chain a centralized manufacturing strategy (up-scaling) may not always result in a lower unit cost of overall, because centralization may have significant implications for both incoming and outgoing supply pathways in terms of increased cost and risk to product quality and customer service. Hence it is essential that all three elements of the supply chain (incoming, manufacturing and outgoing) are considered concurrently so that the costs associated with each can be traded off in-order to identify the optimum physical network configuration i.e., the number and ultimately the location of manufacturing sites. Traditionally this approach is used in logistics network design [29] whereby the costs associated with storage facilities (e.g., warehouses), inventory and transportation are traded to find the minimum total cost network configuration within specified constraints such as demand and customer service requirements. Quality criteria have also been integrated into network optimization models to take account of product degradation in the supply chain [30]. The discussion that follows assumes that, in broad general terms, the more complex a process the more costly it will be per unit of the product, whether that be to source, manufacture or distribute.
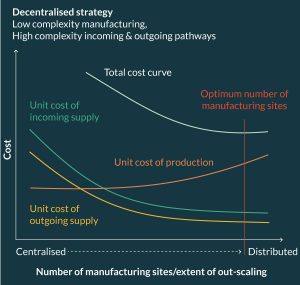
The greater the complexity (and hence cost) of incoming and outgoing supply chain processes the more decentralized (i.e., distributed) the physical supply chain should be, assuming that manufacture of the product can be relatively easily standardized and out scaled, i.e., relatively low complexity of manufacture. Figure 5 presents the cost trade-off between procurement, manufacturing and distribution against increasing decentralization in manufacturing for a product categorized as H-L-H (High complexity incoming pathway, Low complexity manufacture, High complexity outgoing pathway).
Such a product would generate high procurement and distribution costs in a centralized configuration due to the complexity of preserving source material and final product quality as well as the higher logistics costs associated with specialist transportation. In other words, it would be expensive to design incoming and outgoing processes that preserve a labile product as it is moved through a physical supply chain that adopts a centralized manufacturing strategy. However, if there is low complexity in manufacturing and the total supply chain cost of achieving product comparability in an out-scaled decentralized system is less than the total cost of a centralized system then we can avoid the high costs associated with high complexity in supply i.e., the savings made by reducing the need to design expensive distribution and procurement processes outweighs the additional cost of out-scaling manufacturing. In effect, the supply chain strategy is to take the factory to the clinical environment in a decentralized out-scaled cell therapy network.
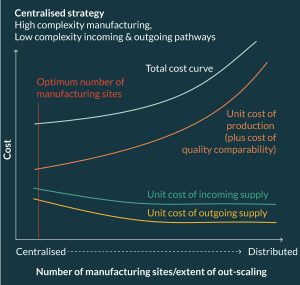
By contrast, the minimum total supply chain cost of a product categorized as high complexity manufacturing, but low complexity incoming and outgoing pathways (category L-H-L) would be firmly tilted toward a centralized manufacturing strategy (Figure 6) that would operate more like a traditional pharmaceutical supply chain. Indeed it is most likely that products categorized L-L-L would also suite a centralized strategy especially if there is opportunity for economy of scale in manufacturing.
However, if all elements of the supply chain are categorized as high complexity (H-H-H) then the minimum cost supply chain configuration would lie somewhere between the two extremes presented above; such intermediary network configurations would comprise a small number of Centers of Excellence serving high density demand clusters, where donors and patients are brought to their closest center for treatment.
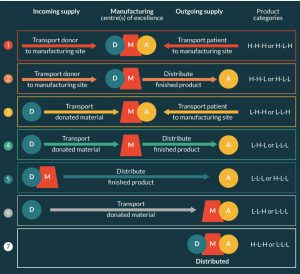
Figure 7 illustrates a range of different network configurations best suited to different product categories as defined by our proposed taxonomy for supply chain selection. For example, the resultant network configuration for HLH products (with relative complexity cost curves illustrated in Figure 5) is represented by Network 7 (Figure 7), i.e., a fully distributed configuration with no movement of product outside the clinical environment. By contrast, Network 4 is best suited to LHL products, i.e., a more traditional configuration in which starting material and finished product are transported to and from a central manufacturing facility. Both Network 4 and 7 are also suited to LLL products, with the choice of network dependent on the potential economy of scale in manufacturing. If the unit cost of production can be reduced by scaling-up manufacturing then the network cost trade-off would point to a centralized configuration, if not then a distributed configuration may be optimal.
Combining products characteristics,supply chain pathway & network configuration into a single supply chain strategy
As the field of cellular therapeutics moves towards large-scale pivotal clinical studies, licensure and adoption by healthcare systems there is urgent and pressing need to properly understand the nature and diversity of the supply chain strategies needed to underpin delivery of this new generation of therapies. Established ‘pharmaceutical’ and ‘healthcare service’ supply chain paradigms will serve to support the development and implementation of only a subset of cellular therapies and lead to somewhat oversimplified debates around the relative utility of ‘allogeneic’ and ‘autologous’ strategies, themselves a misnomer that conflate the source of the starting material with the proportionality between donor and recipient(s) i.e., one to one (which may be autologous or allogeneic) and one to many (which must be allogeneic). Recent publications have identified the complexity of supply chains and lack of standardization in procedures as one of the key challenges facing Advanced Therapy Medicinal Products (ATMPs) in the European market and potentially a contributory factor to the commercial suspension or withdrawal of several of the ATMPs which have achieved Marketing Authorization from the European Medicines Agency [31].
The work of Teng et al. [1] provides a valuable step in proposing a supply chain taxonomy for categorizing cellular therapeutics based on supply and demand uncertainty (Figure 2) but in our view continues to conflate design of the supply chain pathway (i.e., the presence and positioning of decoupling points) with the configuration of the supply chain network (i.e., the degree of centralisation or distribution of manufacturing). In this analysis, we first address the issue of stem cell pathway (Figure 3) and then use this to inform a new taxonomy of the structure of the supply chain network that takes into account the complexity of the incoming, manufacturing and outgoing components of the supply chain (Figures 4–7). Finally it is important to consider that changes to the product (e.g., cryopreservation or an extension to shelf life) can significantly change the supply chain pathway, configuration and costs. Examined from this perspective a number of reflections can be made on the currently licensed cellular therapies in Europe.
Holoclar is an autologous corneal epithelial stem cell product for the treatment of unilateral limbal stem cell deficiency. A corneal biopsy is taken from the patient’s normal eye locally and shipped to a central European manufacturing center where the product is manufactured over 10–14 days and then shipped back for surgical implantation. Thus supply and demand (which are linked) are uncertain, the incoming supply is of high complexity with a labile starting material and long logistic routes, the manufacturing is of medium to high complexity, and the outgoing supply is of high complexity with a labile and delicate product, long return logistics and the criticality of matching supply of the product with surgical scheduling. Neither the incoming nor outgoing supply chains are within the control of the manufacturer. This is therefore a H-H-H type supply chain strategy [32].
Strimvelis is an ex vivo gene therapy of autologous HSC for ADA-SCID – a rare form of congenital immunodeficiency. In principle, the manufacturer faces the same challenges of complex incoming, manufacturing and outgoing supply chains (H-H-H) but because of the ultra-orphan nature of the condition finds it more efficient to transport the donor/patient (one and the same) close to the manufacturing centre, which reduces the supply chain to a L-H-L model and also has the advantage of facilitating administration and follow-up care by a highly specialized (arguably unique) clinical team [33].
Zamloxis is an allogeneic T-cell product genetically modified with a retroviral vector encoding for a truncated form of the human low affinity nerve growth factor receptor (ΔLNGFR) and the herpes simplex I virus thymidine kinase (HSV-TK Mut2) for adult patients undergoing haploidentical HSC transplant for high-risk hematological malignancies. The manufacturers face the same challenges of a HHH type product, but may cryopreserve the starting material (donor T cells) at the procurement or primary processing center, thus allowing easier incoming logistics and decoupling the procurement from the manufacturing in temporal and spatial terms, and then also cryopreserve the finished product following manufacture, again allowing easier outgoing logistics and decoupling the manufacture from the administration. Through the judicious use of decoupling in the supply chain pathway, they can also effectively reduce an H-H-H –to a L-H-L supply chain [34].
As this new generation of products reaches clinical application, it is essential that developers consider the design of the product and the design of its supply chain as inseparable aspects of the same process. We hope that the taxonomy proposed here might help developers structure their thinking around these issues in a more proactive way and help avoid some of the very late stage failures of commercial and clinical application that we have seen thus far.
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Teng CW, Foley L, O’Neil P, Hicks C. An analysis of supply chain strategies in the regenerative medicine industry – implications for future development. Int. J. Prod. Econ. 2014; 149: 211–25. CrossRef
2. Dvorak CC, Gilman AL, Horn B et al. Haploidentical related-donor hematopoietic cell transplantation in children using megadoses of CliniMACs-selected CD34(+) cells and a fixed CD3(+) dose. Bone Marrow Transpl. 2013; 48: 508–13. CrossRef
3. Steinhoff G, Nesteruk J, Wolfien M et al. Cardiac Function Improvement and Bone Marrow Response: Outcome Analysis of the Randomized PERFECT Phase III Clinical Trial of Intramyocardial CD133+Application After Myocardial Infarction. EBioMedicine 2017; 22: 208–24. CrossRef
4. Forbes S, McGowan N, Duncan K et al. Islet transplantation from a nationally funded UK centre reaches socially deprived groups with improved metabolic outcomes. Diabetologia 2015; 58: 1300–8. CrossRef
5. Fraser AR, Pass C, Hakid A et al. Development, functional characterization and validation of methodology for GMP-compliant manufacture of Phagocytic Macrophages: A novel cellular therapeutic for liver cirrhosis. Cytotherapy 2017; 19: 1113–24. CrossRef
6. Baldan V, Griffiths R, Hawkins RE, Gilham DE. Efficient and reproducible generation of tumour-infiltrating lymphocytes for renal cell carcinoma. Br. J. Cancer 2015; 112: 1510–8. CrossRef
7. Vickers MA, Wilkie GA, Robinson N et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. B. J. Haem. 2014; 167: 402–10. CrossRef
8. Fekete N, Rojewski MT, Fürst D et al. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One 2012; 7(8): e43255. CrossRef
9. Mock U, Nickolay L, Philip B et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy 2016; 18: 1002–11. CrossRef
10. Hacein-Bey-Abina S, Pai SY, Gaspar HB et al. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2014; 371: 1407–7. CrossRef
11. Schiroli G, Ferrari S, Conway A et al. Preclinical modeling highlights the therapeutic potential of hematopoietic stem cell gene editing for correction of SCID-X1. Sci. Transl. Med. 2017; 9(411); pii: eaan0820. CrossRef
12. Lane A, Philip LR, Ruban L et al. Engineering efficient retinal pigment epithelium differentiation from human pluripotent stem cells. Stem Cells Transl. Med. 2014; 3: 1295–304. CrossRef
13. Olivier EN, Marenah L, McCahill A, Condie A, Cowan S, Mountford JC. High-Efficiency Serum-Free Feeder-Free Erythroid Differentiation of Human Pluripotent Stem Cells Using Small Molecules. Stem Cells Transl. Med. 2016; 5: 1394–1405. CrossRef
14. Elliott MJ, Butler CR, Varanou-Jenkins A et al. Tracheal Replacement Therapy with a Stem Cell-Seeded Graft: Lessons from Compassionate Use Application of a GMP-Compliant Tissue-Engineered Medicine. Stem Cells Transl. Med. 6: 1458–64. CrossRef
15. Broutier L, Andersson-Rolf A, Hindley CJ et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016; 11: 1724–43. CrossRef
16. Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997; 349: 990–3. CrossRef
17. Turner M, Leslie S, Martin N et al. Towards the development of a global induced pluripotent stem cell library. Cell Stem Cell 2013; 13: 382–4. CrossRef
18. Strauss H, Thomas S, Cesco-Gaspere M et al. WT-1 specific T cell receptor gene therapy: improving TCR function in transduced T cells. Blood Cell Mol. Dis. 2008; 40: 113–6. CrossRef
19. Fisher ML. ‘What Is the Right Supply Chain for Your Product?’ Harvard Business Rev. 1997; 75: 105–16.
20. Blackburn J, Scudder G. Supply chain strategies for perishable products: the case of fresh produce. Wiley Periodicals, Inc., 2009.
21. Payne T, Peters MJ. ‘What Is the Right Supply Chain For Your Products?’ Int. J. Log. Manag. 2004; 15(2): 77–92. CrossRef
22. Godsell J, Harrison A, Emberson C, Storey J. ‘Customer responsive supply chain strategy: An unnatural act?’ Int. J. Log. Res. Appl. 2006; 9, 47–56. CrossRef
23. Christopher M, Peck H, Towill, D. ‘A taxonomy for selecting global supply chain strategies’. Int. J. Log. Manag. 2006; 17(2): 277–87. CrossRef
24. Hilletofth P. Differentiation focused supply chain design. Ind. Manag. Data Syst. 2012; 112(9): 1274–91. CrossRef
25. Hilletofth P, Eriksson D. Coordinating new product development with supply chain management. Ind. Manag. Data Syst. 2011; 111(2): 264–81. CrossRef
26. Mason-Jones R, Naylor J, Towill, D. Engineering the leagile supply chain. Int. J. Agile Manag. Syst. 2000; 2(1): 54–61. CrossRef
27. Hilletofth P. How to develop a differentiated supply chain strategy. Ind. Manag. Data Syst. 2009; 109(1): 16–33. CrossRef
28. Lee HL. Aligning Supply chain strategies with product uncertainties. California Manag. Rev. 2002; 44(3): 105–19. CrossRef
29. Rushton A, Croucher P, Baker P. The Handbook of Logistics and Distribution Management, 3rd Ed. Kogan Page, 2006.
30. Rong A, Akkerman R, Grunow M. (2011). An optimization approach for managing fresh food quality throughout the supply chain. Int. J. Prod. Econ. 2011; 131(1): 421–9. CrossRef
31. Abou-El-Enien M, Elsanhoury A, Reinke P. Overcoming challmeges facing Advanced Therapies in the EU market. Cell Stem Cell 2016; 19: 293–7. CrossRef
32. European Medicines Agency. Public Assessment Report for Hololar (2017):Website
33. European Medicines Agency. Public Assessment Report for Strimvelis (2017): Website
34. European Medicines Agency. Public Assessment Report for Zalmoxis (2017): Website
Affiliations
Christine Rutherford1, Jacqueline Barry2, John DM Campbell3 & Marc Turner3
1 Heriot-Watt University, Edinburgh
2 Cell and Gene Therapy Catapult, London
3 Scottish National Blood Transfusion Service, Heriot-Watt Research Park, Edinburgh