Considerations for the bioprocessing, manufacture and translation of extracellular vesicles for therapeutic applications
Cell Gene Therapy Insights 2017; 3(6), 683-694.
10.18609/cgti.2017.066
EXPERT INSIGHT
There is growing interest in the potential and use of extracellular vesicles (EVs) for a range of diagnostic and therapeutic applications. EVs have been shown, in some instances, to mediate the regenerative effects elicited by stem cell therapies. As such, they are being studied to identify the extent to which these extracellular bodies can be employed as a therapeutic entity, and significant R&D activity is underway to further understand their clinical and commercial potential. However, successful translation will first require further characterization and standardization of EV production, as well as addressing some of the major challenges associated with their reproducible manufacture. This includes the capacity to produce EVs at a scale that is both clinically and commercially effective. This article will highlight some of the bioprocessing and manufacturing considerations and challenges associated with the standardized production of EVs.
INTRODUCTION
The next generation of therapeutics will employ the use of whole cells, such as human stem cells or gene-modified cells, for the treatment of acute and chronic conditions. This has resulted in significant research and development activity to establish large-scale production platforms for human stem cells, both pluripotent (e.g., embryonic and induced pluripotent) and multipotent (mesenchymal and hematopoietic). Whilst there have been numerous clinical trials involving the use of human mesenchymal stem/stromal cells (MSCs) and general recognition that the cells are safe to administer and elicit a therapeutic response, it is unclear as to the underlying mechanism by which the cells induce a therapeutic effect. Evidence suggests that rather than engrafting and integrating with the host tissue as originally expected, these multipotent cells primarily exert their therapeutic effects via the secretion of trophic factors and extracellular vesicles (EVs) into the surrounding environment [1,2] . As such, there has been growing interest in the role of EVs, with these secreted extracellular bodies potentially representing the active pharmaceutical agent for MSCs. Studies have been conducted demonstrating the therapeutic potential of EVs across a wide range of different clinical applications including inflammation, cardiovascular diseases, wound healing and hypertension [3–5] . In view of this potential, efforts are now being undertaken to explore the standardization and manufacturability of EVs at a scale that is both clinically and commercially relevant.
THE ROLE OF EXTRACELLULAR VESICLES
Amongst the complex mixture of factors comprizing the MSC secretome, vesicles are considered particularly significant since they have been shown to mediate endocrine and paracrine signaling in a number of tissues [6] . Vesicles can be differentiated by their size, molecular content and mechanism of biogenesis. Within the majority of biofluids studied thus far, there exists a heterogeneous mixture of vesicles that range in size from 30 to 2000 nm (Figure 1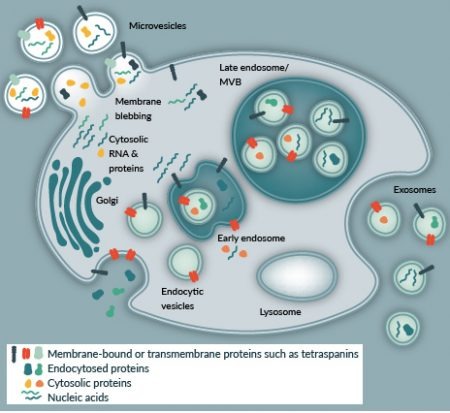
There are considerable advantages in a shift towards an EV-based approach to regenerative medicine when compared with current cell-based approaches. Perhaps the most appealing prospect is that EVs can be fully characterized before administration with no risk of transformation or immunogenicity (at least for stem cell-derived exosomes). The lack of immunogenicity is significant, as it means that like MSCs, the EV source does not strictly have to be autologous. The presence of a bilipid membrane that confers added stability within the circulation, combined with a natural homing capacity can be exploited to stably deliver therapeutic molecules to damaged or diseased sites within the body. The cell-derived lipid membrane also allows for simple and effective loading of EVs with molecules such as RNAs to enhance their therapeutic efficacy, while their protein component limits rapid clearance from the circulation [9] . The natural physiological role of these small particles in cell–cell communication, both local and distant, means that they are naturally tailored for drug delivery, and as such lend themselves to the delivery of a variety of molecular factors to promote tissue regeneration. If the mode of action (MoA) is established and EVs can be reproducibly manufactured at scale, they have the potential to make a significant impact as a biologic.
CLINICAL & COMMERCIAL APPLICATIONS OF EVS
To date, several clinical trials have sought to evaluate the efficacy of EV-based therapies. The majority of trials have applied EVs as anti-cancer agents, where EVs isolated from patients were pre-activated or administered in combination with anti-tumor peptide antigens raised against the cancer cells and then reintroduced to patients to initiate an immune response that specifically targeted the tumor. Such an approach has been applied to target metastatic melanoma [10] , colorectal cancer [11] and non-small-cell lung cancer [12] . These trials have shown initial promise with prolonged tolerance to EV administration reported and only mild inflammatory responses documented as a side effect.
From a regenerative perspective, the number of EV-based clinical studies is steadily increasing as a greater understanding of the role EVs play in a regenerative context is developed. The significant regenerative effects of MSC-derived exosomes first came to the attention of the scientific community in 2010 when they were successfully applied in a murine model of myocardial ischemia/reperfusion injury, promoting neoangiogenesis with a subsequent reduction in the area of infarct-related damage [13] . Subsequent studies have confirmed that the positive effects of stem cell therapies following ischemia are largely a result of vesicles that orchestrate regeneration through the delivery of a biological cargo, such as micro-RNAs that include miR-146a [14] .
Most recently, a Phase 1 clinical trial has been established to investigate the effects of EV-rich plasma for enhanced cutaneous wound healing (NCT02565264). Platelet-rich plasma (PRP) has long been observed to provide significant therapeutic benefits for a multitude of ailments including chronic tendon injury, fractures and sports-related muscle injury. However, due to donor-variation and a lack of understanding of the specific MoA, PRP is difficult to standardize. Exosomes derived from PRP have previously been shown to deliver many of the principal growth factors thought to be, in part, responsible for it’s therapeutic effects [15] . To the authors’ knowledge, this represents the only active clinical trial applying EVs for the purpose of tissue healing/regeneration.
In addition to the aforementioned clinical trials, EVs have been applied with successful outcomes in animal models of acute kidney toxicity [16] ; neurological disorders such as multiple sclerosis [17] and sciatic nerve injury [18] ; and gastrointestinal diseases such as induced colitis [19] and drug-induced inflammatory bowel disease [20] . Despite the relatively recent emergence of EVs as a potential therapeutic intervention, there are now multiple companies established with a focus on commercializing EVs for clinical and/or diagnostic use (Table 1) . These include EVs from a range of different cell sources and for the treatment of a range of different clinical indications.
| Table 1: Companies involved in EV R&D for therapeutic or diagnostic applications. | |
|---|---|
| Company | Cell source |
| Capricor Therapeutics | Cardiosphere-derived cell |
| Codiak Biosciences | Mesenchymal stem cells |
| Creative Medical Technologies Holdings inc. | Amniotic fluid-derived stem cells |
| Evox Therapeutics | Dendritic cells |
| Exogenus Therapeutics | Umbilical cord blood |
| Exosome Diagnostics | Serum/plasma and urine samples |
| Exosome Sciences | Plasma samples |
| Exosomics Siena SpA | Prostate and colorectal cancer cells |
| Kimera Labs | Amniotic fluid-derived stem cells |
| ReNeuron | CTX neural stem cell |
| RoosterBio | Mesenchymal stem cells |
Other technologies and approaches inspired by exosomes include exosome-mimetic nanovesicles, which have demonstrated the ability to deliver chemotherapeutics to the tumor tissue after systemic administration [21] . Through the use of a serial extrusion method with multiple filters of reducing pore size, monocytes and macrophages were broken down to produce the nanovesicles. The authors suggest that such nanovesicles share similar properties with exosomes but have a 100-fold higher production yield [21] . This approach of using cell membrane-derived particles for targeted therapeutic delivery has been an increasing area of research focus [22] . Gao and colleagues developed a system to make cell membrane nanovesicles, which employed nitrogen cavitation to disrupt activated neutrophils [23] . The authors demonstrated the nanovesicles could selectively bind inflamed vasculature and that the administration of the vesicle with TPCA-1 resulted in the significant reduction of acute lung inflammation in mice [23]. More recently, the authors have suggested that there is a 16-fold increase in the production of EVs via nitrogen cavitation compared to naturally secreted EVs and are easier to scale-up than traditional EV production methods[24].
BIOPROCESSING & MANUFACTURE OF EVS
Whilst the clinical utility of EVs in a regenerative context is becoming more apparent, there are numerous challenges to be addressed before EVs can represent a real clinical alternative. These include reproducibility and isolation, storage, scalability (methods for mass production required to achieve clinical scale), characterization, safety and regulation.
Reproducibility & isolation
Reproducibility is dependent on several factors that begin at the donor and cellular level. Since the properties of EVs is dependent on the cells from which they originate and the conditions in which these cells are maintained, it is of critical importance that these variables are standardized to provide a reproducible population of EVs that elicit an optimal therapeutic effect. Donor-to-donor variation is likely to impact on the clinical efficacy of a particular EV therapy. In order to reduce the risk of variation it is important that the MoA of each EV therapy is understood so that it can be standardized and its therapeutic effects reproducible.
Perhaps one of the most pertinent questions within the field is how these valuable biological particles can be isolated at scale in a way that retains functionality and minimizes the inclusion of non-vesicular contaminants, such as membrane fragments and extracellular proteins. EVs are most typically characterized based on their size and protein/lipid content. Current isolation methods have varying influence on the recovery of EV protein and RNA yield and modern solutions will likely be required to generate clinical grade EVs [25,26] . Many recent examples have been published describing the merits and disadvantages of several distinct methods for the isolation of EVs from a diverse range of samples, which includes culture medium, urine, plasma and other body fluids [27] .
Of the multitude of isolation methods reported in the literature, differential ultracentrifugation remains the oldest and most widely applied method. This technique selectively sediments components of interest based on their size and density. However, ultracentrifugation often results in a high degree of variation [28] . Furthermore, it is a lengthy process that provides relatively low yields of between 5 and 25% of the initial starting yield [29] . One way to increase the purity of vesicle fractions is through sucrose gradient centrifugation, which separates vesicles based on their varying flotation densities. The smallest vesicles (exosomes) are recorded to have flotation densities of 1.08–1.22g/mL on sucrose, Optiprep or iodixanol gradients [30] . The advantage of such methods is that they are less prone to capture contaminating cell debris. The downside is that these methods are labor intensive and not suited for high-throughput applications.
More rapid methods include size-exclusion chromatography (SEC) or EV precipitation. Precipitation exploits the differential solubility of EVs in different solvents, such as poly-ethylene glycol (PEG) or PRotein Organic Solvent PRecipitation (PROSPR). A range of commercial isolation kits have recently become available that are designed to sediment exosomes at lower speeds by precipitation with PEG or similar substances. These include the Total Exosome Isolation Kit (LifeTechnologies, USA) and ExoSpin Exosome Purification Kit (Cell Guidance Systems, USA). As such, this method is advantageous in some respects because it does not require specialist equipment, minimizes the risk of damage induced by high centrifugal force and reduces labor. However, it is comparatively expensive when compared with ultracentrifugation and may allow the precipitation of non-exosomal debris that will interfere with the detection of EV markers [31] . This will likely hinder efforts to standardize EV-based therapies [32] . SEC appears to be a promising method that is reported to enable the isolation of a highly pure population of EVs within a defined size-range, while not significantly impacting the downstream analysis of EV surface marker proteins. This method has been employed by Böing and colleagues who were able to isolate EVs with a diameter of >70 nm from platelet-free supernatant of platelet concentrates without co-isolation of protein aggregates [33] . The group successfully demonstrated the technique’s potential as a single-step unit operation for efficient EV isolation. Moreover, SEC is an established downstream processing technique for the purification of intracellular proteins in the biopharmaceutical industry, making it an established and attractive isolation method for EVs. However, the technique has only recently been considered for the EV isolation and additional investigations are required to understand the unit operations efficiency at larger scales.
Other techniques that have been used in the biopharmaceutical industry for the purification of biologics have also been considered for the isolation of EVs; this includes tangential flow filtration (TFF), which has been used for isolation and concentration of EVs in conjunction with other process steps. Indeed, TFF has been used in conjunction with ultracentrifugation for isolation of therapeutic EVs in clinical trials [10,12] . Whilst filtration (both ultra- and nano-filtration) are established techniques and used widely in biopharmaceutical production with proven scalability, current processes employing filtration for EV isolation and concentration requires the use of sequential filtration steps or other purification methods and as such, are incapable of independently isolating EVs. Nevertheless, a recent study demonstrated that ultrafiltration followed by liquid chromatography resulted in a significantly higher yield of EVs when compared with ultracentrifugation, the current gold standard for EV purification [34] . The combination of ultrafiltration and liquid chromatography represents a more capable process with respect to both scalability and isolation of EVs from more complex stem cell media [34] .
Affinity-based selection allows for the isolation of EVs based on their surface protein profile. This can be achieved using magnetic or non-magnetic approaches that incorporate antibodies raised against proteins identified on the EV membrane, such as the tetraspanin proteins CD9, CD63 or CD81. However, since EVs are derived from the cell membrane there is considerable overlap in the surface protein profiles of each and, as yet, no specific marker has been identified to discriminate EVs. Such methods are also typically low throughput and cannot be affordably scaled to produce a commercially and clinical viable product.
Storage
Despite growing interest in the study and application of EVs for diagnostic and therapeutic applications, little information is currently available concerning their storage. It has been suggested that different storage conditions may have a negative influence on the RNA contained within EVs as well as the overall profile of these nano-particles. Zhou and colleagues have previously shown that optimal recovery rates (86%) were best obtained for urinary exosomes if stored at -80°C in the presence of protease inhibitors such as sodium azide, phenylmethane sulfonyl fluoride and leupeptin [35] . The exosomes remained intact and functional with no significant loss of associated protein markers, even when stored at -80°C for seven months. However, further research is required to validate these findings for EVs derived from stem cells. The fact that EVs appear to be stable at -80°C will also reduce costs associated with the specialized cryo-storage of cell-based therapies.
Scalability
Manufacturing high numbers of EVs from human primary stem cells is likely to prove a challenging task. This is primarily due to the fact that stem cell properties can vary if cell density, passage and culture conditions are not kept consistent. Stem cells lose their ‘stemness’ with increasing passage and it stands to reason that the therapeutic effects of EVs isolated from these cells will also be heavily constrained by the number of population doublings the parent cell has undergone. Potency studies will be required to accurately define the window of passage within which stem cells generate therapeutically effective vesicles and if this can be enhanced through the incorporation of agonists within the culture environment. If scaling of EVs for clinical and commercial purposes is to be satisfied, current large-scale manufacturing methods for cell expansion will need to be optimized and an optimal cell source identified. It may even prove necessary to genetically modify the cell source in order to maintain stem cell proliferation, and thereby allow production of EVs that is less restricted by passage. Since EVs are cell-derived products that contain no replicative material of their own, it is likely that changes can be made at a cellular level and are not necessarily propagated within the EVs.
Another significant challenge in the large-scale production of EVs is the inherent difficulties associated with their current manufacture and purification. Although a range of purification options are being considered (discussed above), the current production of EVs involves large-scale cell culture in often complex media formulations, which can contain significant levels of unwanted proteins and other complex macromolecular contaminants. This is particularly the case for processes that use fetal bovine serum (FBS) or human platelet lysate (HPL). Whilst there is an industry-wide focus on using chemically defined cell culture media, it is a significant scientific and technical challenge to adapt an existing FBS/HPL process to a serum-free one.
A process for optimal EV production from the cell source must also be defined, identifying appropriate medium compositions and reagents to facilitate both optimal EV yield and function. This includes understanding whether a batch, fed-batch or continuous process is more appropriate for EV production without impacting on cell proliferation. A range of different bioreactor platforms as well as the operational parameters for such systems will need to be considered to achieve scaling demands whilst maintaining EV function. At present, hollow fiber and packed-bed bioreactors have been used for the continuous production of highly concentrated EVs at scale. Hollow fiber reactors that apply a fiber-base cartridge with a molecular weight cut-off are considered advantageous as this enables the diffusion of nutrients and waste products while retaining the therapeutically valuable EVs [36] . However, stirred-tank bioreactors are also likely to be considered as a key platform for manufacture given the legacy of using such systems for biologics production [37] as well as the increasing propensity to use such platforms for adherent cell cultures in conjunction with microcarriers [38–40] . Stirred-tank bioreactors can be operated with spin filters to avoid the removal of cells/microcarriers during the process whilst facilitating the addition and removal of metabolites, growth factors and other secreted vesicles in a continuous fashion. Moreover, stirred-tank bioreactors have proven scalability and a rich heritage for the production of biologics, making adoption by biopharmaceutical companies which such platforms more likely.
Scalability will also be determined by the nature of the cell source itself. Whilst there is a trend toward stem cell-derived EVs, most of which are adherent cells by nature, there are other non-adherent stem cells (e.g., hematopoietic) and immunological cell types that may be considered for EV production and are suspension by nature, or could be genetically engineered to proliferate in suspension cultures. As such, platforms that are specifically designed for adherent cells such as hollow-fibre bioreactors will not be considered and there will be a focus on employing existing platforms that are used to manufacture suspension-based processes such as stirred-tank bioreactors.
Characterization, safety & regulation
A common challenge across the cell and gene therapy field is the lack of effective and reproducible potency assays. This is similarly a significant barrier to effective translation for EVs, whereby the lack of both in vitro and in vivo assays may hamper progress in the field. However, given the need for such assays, this is now a major focus across the industry and it is expected that significant developments will emerge as we develop a better understanding about the biodistribution of cells and EVs and their mode of action.
A number of safety and regulatory requirements need to be satisfied before the pharmaceutical manufacturing and clinical application of EV-based therapies can be realized. Given the infancy of the R&D activity for EV-based therapeutics, there are understandably no assays for safety testing, and limited information about localization and biodistribution profiles. There must also be consideration of the safety of the cell source from which the EVs are derived. However, given the similarities with biopharmaceutical production, significant learning can be applied from the sector. From a clinical perspective, aside from issues described earlier relating to a lack of understanding of the MoA, both dose finding and toxicity studies need to be satisfied, and immune and tumorigenic response to EVs fully evaluated. From a regulatory perspective, it will be important to define whether EVs represent the active drug component or whether they primarily serve as the delivery vehicle for a drug. As such, significant improvements are required with respect to EV characterization and standardization. This was recently outlined in a position paper by the International Society for Extracellular Vesicles and the Society for Clinical Research and Translation of Extracellular Vesicles Singapore [41] . It is likely that EVs will be characterized similarly to biologics and regulated by the regulatory agencies as such [41] . However, the challenge remains in identifying and understanding the active ingredient or excipients, hence the characterization challenge. As with cell-based therapeutics, it will be important to engage with the regulators from the outset as it may be the case that specific EV-based therapeutic regulations are required [41] .
TRANSLATIONAL INSIGHT
There is a growing body of evidence demonstrating the potential of EVs for both clinical and diagnostics applications. However, as with the cell therapy field, it is important that significant emphasis is placed on understanding the fundamental MoA of EVs so as to begin addressing some of the characterization, standardization and manufacturing challenges. This also involves understanding with respect to the scalable production of both the cell source as well as the fundamental bioprocessing conditions required to enable the reproducible production and purification of therapeutically relevant EVs.
FINANCIAL & COMPETING INTERESTS DISCLOSURE
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
REFERENCES
1. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell2011; 9(1): 11–5.
CrossRef
2. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013; 9(10): 584–94.
CrossRef
3. Lener T, Gimona M, Aigner L et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J. Extracell. Vesicles 2015; 4: 30087.
CrossRef
4. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015; 40: 82–8.
CrossRef
5. Riazifar M, Pone EJ, Lötvall J, Zhao W. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. Annu. Rev. Pharmacol. Toxicol. 2017; 57: 125–54.
CrossRef
6. Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 2013; 44(1): 11–9.
CrossRef
7. Kinoshita T, Yip KW, Spence T, Liu FF. MicroRNAs in extracellular vesicles: potential cancer biomarkers. J. Hum. Genet. 2017; 62(1): 67–74.
CrossRef
8. Fuster-Matanzo A, Gessler F, Leonardi T, Iraci N, Pluchino S. Acellular approaches for regenerative medicine: on the verge of clinical trials with extracellular membrane vesicles? Stem Cell Res. Ther. 2015; 6: 227.
CrossRef
9. Jiang L, Vader P, Schiffelers RM. Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017; 24(3): 157–66.
CrossRef
10. Escudier B, Dorval T, Chaput N et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med. 2005; 3(1): 10.
CrossRef
11. Dai S, Wei D, Wu Z et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008; 16(4): 782–90.
CrossRef
12. Morse MA, Garst J, Osada T et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005; 3(1): 9.
CrossRef
13. Lai RC, Arslan F, Lee MM et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010; 4(3): 214–22.
CrossRef
14. Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014; 2(5): 606–19.
CrossRef
15. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017; 7(1): 81–96.
CrossRef
16. van Balkom BWM, Pisitkun T, Verhaar MC, Knepper MA. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int. 2011; 80(11): 1138–45.
CrossRef
17. Pusic AD, Pusic KM, Kraig RP. What are exosomes and how can they be used in multiple sclerosis therapy? Expert Rev. Neurother. 2014; 14(4): 353–5.
CrossRef
18. Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013; 61(11): 1795–806.
CrossRef
19. Kang CS, Ban M, Choi EJ et al. Extracellular vesicles derived from gut microbiota, especially Akkermansiamuciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One 2013; 8(10): e76520.
CrossRef
20. Cai Z, Zhang W, Yang F et al. Immunosuppressive exosomes from TGF-1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2012; 22(3): 607–10.
CrossRef
21. Jang SC, Kim OY, Yoon CM et al. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013; 7(9): 7698–710.
CrossRef
22. Yurkin ST Wang Z. Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery. Nanomedicine (Lond) 2017; 12(16): 2007–19.
CrossRef
23. Gao, J, Chu D, Wang Z. Cell Membrane-formed Nanovesicles for Disease-Targeted Delivery. J. Controlled Release 2016; 224: 208–16.
CrossRef
24. Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 2017; 135: 62–73.
CrossRef
25. Rani S, O’Brien K, Kelleher FC et al. Isolation of exosomes for subsequent mRNA, MicroRNA, and protein profiling. Methods Mol. Biol. 2011; 784: 181–95.
CrossRef
26. Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol. Biol. 2011; 728: 235–46.
CrossRef
27. Keller, S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011; 9: 86.
CrossRef
28. Livshits MA, Khomyakova E, Evtushenko EG et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015; 5: 17319.
CrossRef
29. Lamparski HG, Metha-Damani A, Yao JY et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 2002; 270(2): 211–26.
CrossRef
30. Raposo G, Nijman HW, Stoorvogel W et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996; 183(3): 1161–72.
CrossRef
31. Gamez-Valero A, Monguió-Tortajada M, Carreras-Planella L et al. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016; 6: 33641.
CrossRef
32. Oosthuyzen W, Sime NE, Ivy JR et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J. Physiol. 2013; 591(23): 5833–42.
CrossRef
33. Böing AN, van der Pol E, Grootemaat AE et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014; 3: 10.3402/jev.v3.23430.
CrossRef
34. Nordin JZ, Lee Y, Vader P et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 2015; 11(4): 879–83.
CrossRef
35. Zhou H, Yuen PS, Pisitkun T et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006; 69(8): 1471–6.
CrossRef
36. Watson DC, Bayik D, Srivatsan A et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 2016; 105: 195–205.
CrossRef
37. Nienow AW. Reactor engineering in large scale animal cell culture. Cytotechnology 2006; 50(1-3): 9–33.
CrossRef
38. Rafiq QA, Brosnan KM, Coopman K et al. Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol. Lett. 2013; 35(8): 1233–45.
CrossRef
39. Jesson HE et al. Storage and Delivery of Stem Cells for Cellular Therapies, in Stem Cell Manufacturing. Cabral JMS et al. (Eds). 2016; Elsevier, Boston, 233–64.
CrossRef
40. Eibl R. Mass production of human mesenchymal stem cells: an approach based on stirred single use bioreactors. In: Scale-up and manufacturing of cell based therapies IV. 2015; San Diego, CA, USA.
41. Reiner AT, Witwer KW, van Balkom BWM et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017; 6(8): 1730–9.
CrossRef
AFFILIATIONS
Owen G Davies1,2& Qasim A Rafiq3
1School of Sport, Exercise and Health Sciences, Loughborough University, UK
2School of Chemical Engineering, University of Birmingham, UK.
3Department of Biochemical Engineering, University College London, UK
* Corresponding authors: q.rafiq@ucl.ac.uk
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.