Scale-up of platelet production from human pluripotent stem cells for developing targeted therapies: advances & challenges
Cell Gene Therapy Insights 2017; 3(9), 701-718.
10.18609/cgti.2017.068
Without question, the future of regenerative medicine is in the scalable production of transfusable human tissues for therapeutic use. Platelets will be among the first of these stem-cell based therapeutic tissues to be developed and adopted for clinical use, most notably because they are anucleate and can be safely irradiated to substantially reduce the risk of teratoma development and other cell contaminants. Furthermore, platelets are short-lived, well characterized, easily transplanted, are not required to be autologous, and support a larger than $20 billion per year global market that relies entirely on human volunteer donors. While we are within a decade of realizing the potential of stem cell-based therapeutics, this was not obvious only several years ago. This article identifies the major logistical challenges associated with commercially scaling platelet production for therapeutic use and our experiential insights into translating this technology to the clinic.
While platelets are primarily responsible for clot formation at sites of active hemorrhage, it is becoming increasingly apparent that they also play significant roles in wound healing, angiogenesis and innate immunity [1–6]. Among the stem cell-based therapeutic landscape, platelets are an ideal early entrant because they: are in high clinical demand due to their short inventory shelf-life [7]; are anucleate and can be safely irradiated to kill any contaminating nucleated cells, thereby reducing the risk of teratoma development [8,9]; do not require HLA or blood type matching for the majority (>90%) of platelet transfusions, which will facilitate large-scale manufacture of allogeneic human pluripotent stem cells (hPSCs) for off-the-shelf therapy (Box 1); are short-lived and well characterized, which will simplify projected clinical trials [10]; are easily transplanted [11]; and would benefit tremendously from sterile manufacture, as current donor-based platelet transfusions are inherently susceptible to bacterial and viral contamination [12,13].
Current platelet demand exceeds supply by ~20% [14], and unmet demand is expected to more than double by 2022 due to a growing and aging population requiring more platelet-based procedures, new medications that reduce platelet counts, and the expansion of existing uses of platelet transfusions to improve healing time [15]. In the USA, the blood market effectively operates as an oligopoly, with the American Red Cross and America’s Blood Centers each controlling a little less than half of the market and HemeXcel as the third major blood provider. There are numerous other potential markets for platelets and their growth factors that are currently constrained by lack of access to platelets. For example, platelet growth factors can be used for cell culture, tissue regeneration, wound healing, drug delivery, and there is even work in the cosmetics industry using platelets for skin rejuvenation [16–28]. Platelet BioGenesis is addressing this need by establishing a scalable, current good manufacturing practice (cGMP)-compliant commercial platform to make safe and functional human platelets [29]. In this article, we describe the challenges in bringing hPSC-platelets from the research phase through to commercially viable products. We lay out best practices for overcoming these obstacles, ensuring regulatory compliance, establishing a scalable process, and securing freedom to operate. We conclude with translational insights into the future of regenerative medicine and the market expansion of stem cell-derived platelet products. While all platelets are hPSC-derived, for the purposes of this article hPSC-derived platelets will refer to platelets generated outside the human body by industrial cell culture.
| Box 1 |
|---|
| Platelets synthesize class I HLA (but not class II). While platelets absorb soluble HLA antigens from plasma, leading to relatively higher numbers of class I HLA antigens on their surface as compared to red cells and granulocytes, the incidence of HLA antibodies following platelet transfusion is low (10–20%) [71,72]. Interestingly, >50% of patients with HLA antibodies fail to manifest refractoriness, such that the incidence of HLA-mediated refractoriness following platelet transfusion is only 3–5% [71]. |
| Serum-free ex vivo production of platelets should result in lower numbers of class I HLA antigens on their surface potentially resulting in a lower incidence of HLA-refractoriness still. While this prediction will need to be borne out in the data, it argues strongly against the need to invest in the production of HLA-null (universal) platelets as a first-tier product. |
COMMERCIALIZATION CHALLENGES
There is a tremendous amount of variability among donated platelets and the various quality assessment metrics that are used to gauge platelet function [30]. Despite this fact, there has been a historical presumption that donor-derived platelets ‘work’, and there is no single gold-standard assay to assess donor platelet unit quality and function ahead of transfusion. Instead, current US Food and Drug Administration (FDA) requirements regarding platelet unit preparation focus on storage temperature, pH, residual leukocyte count and bacterial/viral contamination rate. To license platelets based on post-storage radiolabeled autologous platelet viability measurements in normal subjects, the FDA requires that the lower post-storage 95% confidence limits (LCLs) for platelet recoveries are ≥66% and survivals are ≥58% of the same subject’s radiolabeled fresh recoveries and survivals, respectively [31]. While donors volunteer platelets, procurement of platelet units is not free. Blood bankers, who are the key decision makers for platelet orders and distribution, choose their platelet supplier based on availability and price (Box 2). Therefore, in addition to demonstrating non-inferiority relative to donor platelets, hPSC-derived platelets need to be cost-competitive to warrant commercial adoption.
| Box 2 |
|---|
| A leukoreduced, irradiated apheresis platelet unit in the United States costs approximately $1000 when accounting for the cost of discarded units. Leukoreduced (non-rradiated) platelet units are marginally cheaper, averaging just under $750 per unit and comprising an additional ~50% of all platelets transfused. Prices vary greatly depending on supply and demand [73–76]. |
The improved safety, projected ‘on-demand’ availability, functional definition of product, and lower inter-unit variability constitute the major value propositions that will drive market adoption of hPSC-derived platelets over donor platelets. Additionally, the reduced inter-unit variability will substantially empower physicians to begin predicting how donors will respond to platelet transfusions. Commercial development of hPSC-derived platelets should therefore strive to leverage the value propositions of donor independence (sterile, safe, and scalable products) and on-demand manufacturing capability by:
- Advancing a donor-independent, cGMP-compliant hPSC line rather than developing processes around research grade or animal lines that cannot be advanced clinically, or relying on primary CD34+ stem cells that carry the same availability, sterility and cost limitations as donor-derived platelets.
- Establishing a consistent differentiation protocol that avoids serum or feeder-cells, which would likely make regulatory approval substantially more challenging and add variability and contamination risk to the product.
- Developing an industrial process during early stages of development that will scale to meet future clinical and then commercial demand for hPSC-derived platelets, so that the process does not need to be redeveloped at each stage.
- Prioritizing first quality and then cost, so that hPSC-derived platelets will meet regulatory requirements and result in a product that is commercially viable.
The tradeoff between focusing on quality and cost is the principal challenge for regenerative medicine and a significant barrier to creating a scalable and profitable business in this space. While regenerative medicine is expected to ultimately improve patient safety and reduce overall costs, PSC-derived products are likely to be more expensive in the short term. It will therefore require all stakeholders working together to support a commercial path to market for early regenerative medicine technologies such as platelets, by supporting transformative advancements in this space and providing reimbursement rates that reflect these long-term benefits.
REGULATORY COMPLIANCE
Current Good Manufacturing Practice comprises a set of standards that are set forth by the FDA that describe ways that manufacturing processes and facilities must be designed, overseen, and operated. Observation of cGMP during the manufacture of hPSC-derived therapeutics ensures that the consumer receives products of the correct identity, strength, quality and purity. In order to be cGMP-compliant, the responsibilities of the manufacturer of an hPSC-therapeutic include obtaining appropriate quality raw materials, establishing robust operating procedures, establishing strong quality management systems, detecting and investigating product quality deviations, and maintaining reliable testing laboratories.
For hPSC-derived platelet production, the product will need to meet a number of regulatory requirements in order to gain investigational new drug (IND) approval [35]. This includes compiling a description of the:
- Proposed indication
- Dosage and route of administration
- Product description and chem-istry manufacturing controls (CMC), including:
- iPSC line derivation
- Master cell bank production
- Megakaryocyte production
- Platelet production4.
- Product characterization, including:
- iPSC cell line characterization
- Megakaryocyte characterization
- Platelet final product characterization
- Storage characterization
- Description of pre-clinical studies
- Clinical development plan, including:
- Phase 1 clinical study synopsis
- Phase 2 clinical study synopsis
While this is an ever-changing landscape, there are guiding principles that will need to be considered for advancing hPSC-derived platelets. Most differentiation processes are developed in primary hPSC-lines or animal lines because of accessibility and cost, but it is critical to note that cost or regulatory compliance may prohibit their commercial advancement and pre-clinical validation studies will need to be re-established using a cGMP-compliant line. This is especially true of research-grade PSC-lines, as different PSC-lines will perform very differently using the same differentiation protocol [36,37], which will add substantial time, cost and risk to the research and development strategy.
The potential immunological response to a stem cell generated therapeutic, which could be driven by either the hPSC-line itself or products used during the differentiation process, is equally important to consider. Human leukocyte antigen (HLA) is a polymorphic, cell surface protein that is expressed on the majority of nucleated cells and mediates immunoreactivity to transplanted tissues [38]. If the immune system identifies cells expressing HLA molecules that it deems to be ‘foreign’ within the body, it will mount an immune response that can lead to graft rejection or complications following transfusions or transplants [38]. Therefore, HLA compatibility must be taken into account during the selection of an hPSC-line. This is less of a problem with platelets, but this can be a major issue with other cells/tissues (Box 1). One risk-mitigating approach is to begin with hPSC-lines derived from HLA SuperDonors, which have HLA genetic profiles that make their cells less likely to cause immune complications in platelet recipients [39]. The basis for this is a partial HLA match with most recipients that has been shown to be beneficial in organ transplants [40]. Indeed, hPSCs have been successfully generated from HLA SuperDonors using a proprietary transgene-free and virus-free episomal reprogramming process that utilizes circular DNA vectors to deliver pluripotency genes driving human induced PSC production [41–46]. As this reprogramming method does not rely on integration events, it alleviates major safety concerns about potential use of hPSC-derived products in cellular therapies (e.g., genetic integration of the transgene vector into off-target genes with negative consequences, escape of the virus carrier, etc.) [34,47], and it is recommended for making platelets.
Once the appropriate stem cell line is identified, the differentiation process also needs to be adapted to be cGMP-compliant. Stem cell cultures typically rely on embryonic fibroblast feeder cells that can potentially be contaminated with xenogeneic pathogens, increasing the risk for an immunogenic reaction in humans. Alternatives to animal serum and feeder cells are desirable to avoid the introduction of animal products into human tissue culture, to reduce the harvesting of cells or serum from animal fetuses, and to ensure safe and animal product free conditions for cGMP [28].
Additionally, it is important to ensure that any reagents that are being incorporated into the differentiation protocol are available in cGMP-compliant versions. The use of different versions of reagents (both within and between manufacturers) can cause variability in cell culture outcomes, resulting in the need for additional optimization steps. This can add unnecessary time and cost to the process of translating a stem cell culture process to the clinic. Similarly, it is important to establish supplier relationships in order to ensure that the required products can be made available at clinical or commercial quantities and at a cost that supports the scalability of the process.
In order to ensure that time is not wasted unnecessarily, it is also important to establish a scalable manufacturing process that can be performed in a cGMP-compliant manner. If this is not done initially, the process will need to be redeveloped and approved prior to clinical trials and again at each scale up stage, adding substantial time and cost to the development and approval process. This point was recently made and elaborated upon in a FDA guidance document [48]. This also requires the establishment of supplier relationships that afford access to cGMP-compliant bioreactors at commercial and clinical volumes that support scalability of process in a cost-effective manner.
SCALABILITY OF PROCESS
The regenerative medicine market’s rapid growth is projected to continue and is estimated to exceed $38 billion within 5 years. Stem cell-based therapeutics will require the continuous generation of trillions of hPSCs and their resultant differentiated cells. However, current laboratory-scale tissue culture methods using culture dishes or spinner flasks cannot produce clinical-grade cells at this scale in a robust and cost-effective manner. The outcome is an inability to scale processes linearly, which, along with the cost of cell culture media, constitutes a major limiting factor to market growth. Laboratory scale production needs to be replaced by industrial-scale processes, which means that technology relying on small-scale bioreactors must be redeveloped in larger volume systems that maximize efficiency and yield while minimizing cost.
Platelets are produced by large, polyploid, PSC-derived cells called megakaryocytes that reside adjacent to sinusoidal blood vessels in the bone marrow. During thrombopoiesis, megakaryocytes generate bead-like extensions called proplatelets that enter these vessels and sequentially release platelets into the circulation. Whereas bone marrow megakaryocytes generate >2,000 platelets each within <12 hours, in vitro hPSC-derived megakaryocytes in static culture generate <10 platelets each over 48–72 hours [49]. To improve the efficiency of stem cell based tissue production, we should follow a key guiding principle for regenerative medicine and look to the body for inspiration on how to reproduce physiologic processes in an industrial setting. The overarching goal in this is to identify minimum viable triggers for directed differentiation. In the case of platelet production and achieving increased total platelet yield, anatomical platelet production can be translated into four major phases of industrial cell culture that mimic what occurs in the bone marrow. First, hiPSCs must be expanded in vitro to generate an adequate number or precursor cells. Secondly, hiPSCs must be differentiated into functional megakaryocytes that are themselves capable of platelet production. Thirdly, megakaryocytes must be triggered to produce platelets. Finally, the platelets must be isolated and concentrated into transfusable units (Figure 1).
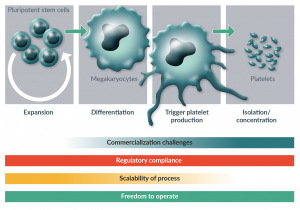
Figure 1: Phases of platelet production and challenges with industrial scale production at each stage.
Industrial-scale production of platelets requires the optimization of four phases cell-culture processes that mimic anatomical platelet production: expansion of hiPSCs to generate adequate numbers of progenitor cells, differentiation of precursor cells into functional megakaryocytes, triggered production of platelets by megakaryocytes, and isolation and concentration of hiPSC-derived platelets. In order to successfully commercialize hiPSC-derived platelet products, freedom to operate and maintenance of regulatory compliance are necessary at each stage of development. Each stage has unique challenges when it comes to achieving scalability of the process and overcoming commercialization challenges.
Many of the challenges in producing stem cell-derived products are illustrated by the production of platelet generating megakaryocyte cells, which constitutes the first two phases of industrial platelet production. Like many hPSC-derived cell types, megakaryocyte generation from hPSCs comprises multiple differentiation steps, which include both adherent and soluble phases and different media requirements. Additionally, the cells are sensitive to shear stress, there are major size differences between the immature and differentiated cells, and there is a need to isolate specific cell intermediates at multiple stages within the process. This requires the serial linking of multiple differentiation stage-specific bioreactors together to enable scaled up industrial production. While complex production processes such as this have been successfully implemented in a few cases (e.g., cardiomyocytes [50,51]), production limitations have prevented the clinical advancement of many other hPSC-derived cell types (including megakaryocytes), and remain a major hurdle in the commercialization of hPSC-derived platelets. Given the complexity of the process and its parallels to the generation of other stem cell derived tissues, solutions developed here are expected to be broadly applicable to general cell culture for tissues that others are working to produce.
While much progress has been made in the development and optimization of these processes to generate functional platelets from hPSCs, up to now this has been performed exclusively at a small scale. The next major challenge in the translational application of hPSC-derived platelets will be to transition from laboratory scale to industrial scale platelet production. One platelet apheresis transfusion unit contains ~3×1011 platelets in 300–500 mL [49]. In the USA, approximately 2 million apheresis-equivalent platelet units are required annually, or approximately 6×1017 platelets [49], which represents a major manufacturing challenge. Ways in which we can begin addressing these needs and optimizing phases three and four of industrial platelet production are two-fold: first, we must achieve greater yields of product cells from progenitors (e.g., we must be able to isolate more megakaryocytes per hPSC and more platelets per megakaryocyte); and secondly, we must develop and optimize the physical infrastructure to support the generation of these numbers of cells and the media necessary to produce them.
To date, most of the platelet bioreactors that have been developed have been custom-made, proof of concept research tools that are low-throughput and only capable of producing platelets at a laboratory scale. During the transition to the fourth phase of industrial scale platelet production, several new approaches will be required during bioreactor development and manufacture to enable the scalability of the production process. Firstly, platelet bioreactors capable of commercial scale production must be manufactured using standardized processes and biocompatible materials that allow for automation. Secondly, it is critical that we establish standardized operational processes and device validation metrics that will meet regulatory requirements and be cost effective, and that the failure rate for manufacture be very low [52]. Within these operational constraints, the following major advancements that still need to be achieved in the development of next-generation millifluidic devices including:
Automation of flow control
Commercial scale platelet bioreactors must be able to be run without direct human supervision. These devices must be capable of running in parallel over continuous process conditions that account for the pressure differentials that are generated in devices large enough to meet projected scale requirements.
Bioreactor computational fluid dynamics modeling
An optimally designed bioreactor should distribute cells uniformly and ensure equal agonist (e.g., shear stress, extracellular matrix protein) exposure of all cells, regardless of their position in the device. This uniformity would allow for conditions to be precisely modulated in order to identify ‘sweet spot’ ranges for individual drivers of platelet production. Computational fluid dynamics modeling within the bioreactor will be necessary to resolve flow lines during device operation and identify actual forces at play on cells at different positions on the device, which could then be modulated to meet these goals.
Use of biocompatible (non-PDMS) scalable materials
PDMS is commonly used for microfluidic device prototyping because many of its properties make it ideal for device development and affordable manufacture [53]. However, it is not suitable for use in commercial biomedical devices and it is critical that we transition to biocompatible FDA-approved thermoplastics during the development of commercially scalable platelet bioreactors. PDMS can absorb small molecules and introduce uncross-linked oligomers from the curing process into solution [54,55], which can result in cytotoxicity and can affect critical cell signaling dynamics and drug concentration studies. PDMS is also unsuitable for high-throughput manufacturing methods such as injection molding, rolling and embossing [56], preventing scalable manufacture. Alternative scalable materials that could be used in next-generation platelet bioreactors could include acrylic, cyclic olefin copolymer, polycarbonate, polyvinyl chloride, polyolefin, polyethylene, polyurethane, polypropylene or graphene oxide.
Scaled design that supports commercial application
In order to perform preclinical studies, including those needed to assess platelet safety and quality in vitro and to perform functional studies in mice (Tables 1 & 2), platelet bioreactors must be scaled up to support the production of at least 3×106 megakaryocytes to produce the required 3×108 platelets [35,49,57]. The bioreactors will need to be scaled up further prior to commercialization to accommodate at least 3×109 megakaryocytes to produce 3×1011 platelets, the equivalent of one apheresis platelet unit, and the millions of platelet units that are needed for use globally. Furthermore, access to platelets today is currently restricted to transfusion patients in major cities in developed countries. There is significant unmet demand by patients in the developing world and from other industries, such as cosmetics and orthopedics, which desire access to the regenerative effects of their high concentration of growth factors. These industries and their platelet demand are expected to grow further upon the availability of hPSC-derived platelets and the development of novel applications of platelet products.
| Table 1: Targeted platelet quality assessment metrics | |||
|---|---|---|---|
| General attribute In vitro assay | Specific attribute | Targeted performance specification Resting | ǂActivated (0.1 U/mL TRAP/1μg/mL CRP/20 μM ADP) |
| Morphology Light Microscopy | Size Shape | 1.5–3.0 μm Discoid | – Circular or flat/spread |
| Cytoskeletal organization Fluorescence microscopy | β1 tubulin F-actin | Cortical coil of 6–8 MTs Central network | Centralization Lamellipodia and filopodia formation |
| Granule content Fluorescence microscopy | PF4 (alpha granule) Serotonin (dense granule) | 40–80 2–7 | Fuse with plasma membrane Release content |
| Ultrastructure Electron microscopy | Membrane conformation Glycogen granules Organelle content Open canalicular system Dense tubular system | Smooth Present 2–8 mitochondria Present Present | Ruffled – – – – |
| Biomarker Expression Flow cytometry | CD41/61 PAC-1 CD42b CD62P CD63 Annexin V (5 mM CaCl2 ) | >80% <20% >80% <20% <20% <10% | >80% ≥80% >80% ≥20% ≥20% ≥10% |
| Aggregation Aggregometer | Aggregation Aggregation amplitude (+10 μg/mL Collagen) | – – | ≥120% slope ≥95% |
| Clot retraction Glass test tube, plasma | Clot retraction (measured at 2 and 24 hours) | - | Grade 3 or higher at 2 hours Grade 4 or higher at 24 hours |
| In vitro assay | ǂActivated (collagen, Fg, vWF) | ǂActivated (collagen, Fg, vWF) | |
| Thrombus formation Perfusion chamber | Extent of thrombus formation – low vs high shear (calciein-AM) | +Whole blood 30 minutes | +PLT-depleted blood 30 minutes |
| In vivo assay | |||
| Circulation time/clearance Infusion into humanized vWF mice | Circulation time Platelet recovery/survival | 8 hours ≥66/≥58% | – – |
Scaled manufacture & assembly of devices
Current production techniques for platelet bioreactors should be modified to introduce scalable design principles that will reduce inter-batch variability during testing and will eliminate the need for device redesign and revalidation in the future. The use of microinjection molding, rolling or embossing techniques will allow for the eventual large-scale reproducible manufacture and automated assembly of devices. However, it is also necessary to allow for flexibility in production to adapt to expected future scientific advances and market changes.
| Table 2: Targeted platelet safety specifications. | ||||||
|---|---|---|---|---|---|---|
| Platelet metabolic activity metrics | Units | Acceptable range | Target | Allowable process failures | ||
| Storage temperature | °C | 22–24 | With continuous gentle agitation during storage | |||
| Actual platelet yield of transfusable component | x1011 | ≥3.0 | 95%/75%* | N = 11** 0 | N = 18** 1 | N = 23** 2 |
| pH at 220C | pH | ≥6.2 | 95%/95%*** | N = 60** 0 | N = 93** 1 | N = 124** 2 |
| Residual leukocyte count | (95% of units) | <5x10 6 | 0 | 1 | 2 | |
| Bacterial contamination rate | ≤1:5000 | 95% | ||||
| * 95% confidence that greater than 75% of the components meet the standard. | ||||||
| ** The sample size numbers can be used in a samploing plan that should be representative of products collected on each machine type in each facility. | ||||||
| *** 95% confidence that greater than 95% of the components meet the standard. | ||||||
Media volume reduction & product concentration
At present, a major bottleneck in all phases of industrial-scale platelet manufacture is cost, which is largely driven by cell culture media. Culture phases typically extend for multiple days and require the optimization of growth factors and daily media exchanges. ‘Batch-feeding’ media change approaches involve siphoning off the majority of the media and replacing it with fresh media daily. This approach is not ideal for stir tanks and other large 3D bioreactors because it requires that the stirrer be stopped for a period of time to allow the cells to settle to the bottom of the tank prior to removal of the old media, making it cumbersome and expensive. Additionally, the shock of the media exchange has unknown biological impact on the cells. An alternative to batch feeding is utilizing a millifluidic perfusion bioreactor which employs real-time sensors to continuously replace media with appropriate supplements to replenish small molecules that are depleted over time in culture [58–60]. Cells are retained in the bioreactor during the media exchange by a porous filter or other cell retention device. Critically, perfusion bioreactors can perform these media exchanges while maintaining low, controllable and uniform shear and pressure on cells. Incorporating media recirculation loops in millifluidic device design will help to minimize operational volume, reduce media/cytokine cost, and further concentrate the cell product. Platelets must remain agitated and in suspension to maintain their functionality, which can be achieved through the introduction of peristaltic pumps [61,62], which have the additional advantage of being able to recapitulate the physiologic pulsatile flow of ~1 Hz [63,64]. We have had success implementing this approach in hPSC-derived platelet production processes, with the potential to expand it to other large-scale, general cell culture efforts [35,49,65].
In parallel to the advances that are being made on technological and process optimization, it is critical that a regulatory strategy is developed in collaboration with NHLBI, NIAID, DoD, BARDA and the FDA. These strategies should establish industry standards for quality and functional assessment of cell-based therapeutics, operational process standards, and bioreactor monitoring methods that will fit into larger manufacturing processes. The FDA does not just approve the product, but also the process, which means that pre-IND validation studies need to be performed using a cGMP compliant process that will remain consistent during clinical-grade product manufacturing. While process changes can be made, this requires reassessment by the regulatory agencies and approval of the change by the FDA prior to implementation into clinical manufacture.
FREEDOM TO OPERATE
In order to successfully commercialize a regenerative medicine technology, it is not only necessary to overcome the scientific challenges presented with the technological development but to also obtain commercial freedom to operate. Ideally, this includes control over the entire supply chain, and for hPSC-derived platelet production this can be consolidated into three critical families of intellectual property (IP) [35]. The first is development of or license to a clinical grade hPSC line for commercial therapeutic use. The second is a cGMP compliant process to generate platelet producing-megakaryocytes that is matched to the clinical grade hPSC line. Third, one must develop a scalable, cGMP-compliant method of triggering megakaryocytes to produce platelets (such as a platelet bioreactor). As the field progresses it should be presumed that at each step within the differentiation process there will exist the opportunity to include additional technologies that can further improve the cost and quality of cells produced. However, these opportunities should be considered carefully in light of the associated licensing expenses that can offset the benefits and can limit the commercial viability of the product. Technology licenses typically contain a number of financial terms, including an up-front fee, milestones payments, annual license payments and royalties. For sufficiently large target markets (such as platelets), the cost of one-time cash payments can be amortized over a very large number of units to become negligible. Royalties present a more substantial issue in regenerative medicine products that are high volume but relatively low margin (like platelets). Even minor royalties can stack up and can quickly consume the profit margin. This will stall product development and destroy significant value that could be shared by all partners and, most importantly, prevent life-saving technology from reaching patients.
In emerging industries such as regenerative medicine, the initial IP filed tends to be very broad and represents ‘foundational’ patents in the space. Ownership of foundational patents gives a significant advantage to first movers, effectively blocking any other entrant from commercializing a competing product. Moving early to obtain freedom to operate (or even better, to control the IP landscape) will ensure a company has an indisputable competitive advantage and creates a path for a project to be profitably financed by private investors. Furthermore, as new discoveries are made that build on the foundational IP, the opportunity set will grow, and these early companies and their investors will reap the rewards of the risks taken.
TRANSLATIONAL INSIGHT
The regenerative medicine industry is still in its infancy, with rapidly evolving technology and near limitless possibilities for its application. This represents a huge opportunity that companies establishing themselves today will be best positioned to capitalize upon. In financial terms, investors should think of backing these technologies as buying an option: there is significant value to the optionality inherent in a young industry – if you invest in teams capable of executing on this optionality. Investors and companies who are late to the game risk missing the opportunity to capture these significant markets (Figure 2). This option value is already being realized in the platelet space, with applications extending beyond the initial focus on tissue regeneration into matters of national security, drug delivery and personalized medicine.
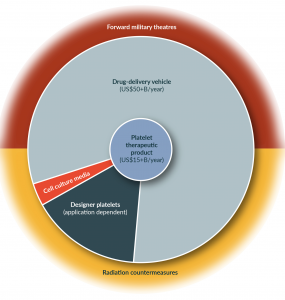
Figure 2: Expansion of platelet-product markets.
The number of potential markets for hiPSC-derived platelet products is rapidly expanding from its initial use as a therapeutic product into other market spaces including drug delivery, cell culture media, personalized medicine, and national security countermeasures. This trend of rapid market expansion will likely also apply to other areas of the regenerative medicine industry.
National defense and security initiatives are a high priority within the USA and represent a large potential market for hPSC-derived platelet products as a radiation countermeasure. Radiation exposure, such as would occur following a nuclear accident or attack, inhibits platelet production. As the world grows smaller it is increasingly likely that the next war will be fought in heavily populated urban centers. Due to the increasing reach of missiles and the growing risk of radiological attack at home, allied countries’ platelet availability is a national security issue. A large radiological event would trigger an immediate demand for platelets that would deplete existing local inventory to treat emergency trauma, followed by a sustained demand for platelets in survivors 6+ days post-exposure. National strategic stockpiles of platelets will become very important as our military’s preparedness gap shifts from the front lines to the 24–48 hours post-incident when affected populations become thrombocytopenic. The USA does not maintain an inventory of platelets in the Strategic National Stockpile and there are no licensed therapeutics that immediately increase platelet counts. The NIH Radiation Countermeasure Program has specifically highlighted the Nation’s platelet supply as a “critical unmet need” [66]. Our ability to bank platelet progenitors and develop on-demand hiPSC-platelet production capabilities will enable the establishment of a strategic national stockpile of hiPSC-platelets that will be critical to meeting this projected need. Along with rapid de novo production of platelets following an attack, frozen pre-megakaryocyte intermediates can be stored for extended periods of time and stockpiled to enable an on-demanded rapid ‘surge’ production of platelets when needed.
Another novel application of hPSC-platelets is as a vehicle for drug delivery. Platelets circulate in the bloodstream and touch every organ in the body, providing them with the potential to serve as part of a versatile, customizable and targetable drug delivery system. Moreover, because of their immunomodulatory and angiogenic functions, platelets are actively recruited by tumors to aid in immune evasion and support their growth and metastasis [67,68]. Proof-of-concept studies have shown that platelets can be used to transport drugs and hone in on cancer cells by becoming engineered to express antibodies [27], becoming tagged with antibodies on their surface [23], or being engineered to express or cultured to ‘take-up’ various antibodies or molecules into their secretory granules [24,26]. Using these methods, platelets could be loaded with therapeutic molecules and directed to the desired tissue (or tumor). The benefits of this type of drug delivery include the ability to deliver molecules to tissues that are traditionally hard to target due to limitations imposed by permeability or retention of the drug, and a reduced need to treat a patient systemically, which often results in unwanted toxicities [69].
A third option is to create ‘designer platelets’ expressing desired characteristics for targeted applications. These tools can be leveraged to generate the specialized outcomes that personalized medicine approaches promise, without the drawbacks that have prevented their commercial implementation (high cost, time intensive, and inability to scale products). Rather than generating custom hPSC lines from individual donors, it is preferential to develop a platform that utilizes cGMP-compliant hPSC lines that are optimized for therapeutic product manufacture. Genetic control of the hPSC lines can then be applied to generate designer products for targeted therapeutics and recipients. For example, designer platelets expressing high levels of clotting factors, e.g. Factor VII, in their granules could enhance clot formation at the site of damage without risk of systemic hypercoagulation. These ‘hair-trigger platelets’ could be targeted for trauma, increasing the effectiveness of platelet transfusion during the first ‘Golden Hour’ following severe traumatic injury. Another potential application for designer platelets is in the treatment of fetal and neonatal alloimmune thrombocytopenia (FNAIT). In this condition, fetal platelets expressing a human platelet antigen (HPA) that their mother does not express are targeted by the mother’s immune system, leading to fetal thrombocytopenia and serious potential complications (including fetal intracranial hemorrhage) [70]. This condition could be treated using designer platelets that have been engineered with a single base pair change, such that HPA (negative) platelets are administered to a HPA positive child after delivery by HPA-negative women, preventing the HPA-associated platelet clearance.
Other hPSC-derived therapeutics will undoubtedly discover similar second-generation opportunities that build on the foundation of their core technology, which is a demonstration of the overall promise of regenerative medicine. If executed properly, the returns from these opportunities can far exceed the initial first generation product envisioned by company founders, both in terms of their ability to help patients and financially. To achieve this vision, we must first establish scalable, cGMP-compliant manufacturing processes that are safe, functional, and cost-competitive. The significant progress that has been made and that we, and others in the platelet space, continue to build upon is a vital model for other regenerative tissue markets to come.
ACKNOWLEDGEMENTS
We would like to thank Jessica F. Olive for her insightful feedback and editing support. We also acknowledge the generous funding support of the NIH (#1R44HL131050-01, #1R43AI125134-01A1, and #1SB1HL137591-01) and the Massachusetts Life Sciences Center (MLSC Ramp Grant).
FINANCIAL & COMPETING INTERESTS DISCLOSURE
J.N.T. and S.M.K are partially supported by NIH grants #1R44HL131050-01, #1R43AI125134-01A1, and #1SB1HL137591-01. J.N.T. and S.M.K. are paid employees of, have financial interest in, and are founders of Platelet BioGenesis, a company that aims to produce donor-independent human platelets from human-induced pluripotent stem cells at scale, and is an inventor on their patents. J.N.T. is an unpaid affiliate of Brigham and Women’s Hospital and Harvard Medical School. The interests of J.N.T. and S.M.K. were reviewed and are managed by Platelet BioGenesis in accordance with their conflict-of-interest policies.
REFERENCES
1. Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science 2010; 328(5978): 562–4.
CrossRef
2. McMorran BJ, Marshall VM, de Graaf C et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 2009; 323(5915): 797–800.
CrossRef
3. Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nature Immunol. 2013; 14(8): 785–92.
CrossRef
4. Battinelli EM, Markens BA, Italiano JE Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 2011; 118(5): 1359–69.
CrossRef
5. Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015; 29(3): 153–62.
CrossRef
6. Etulain J, Negrotto S, Tribulatti MV et al. Control of angiogenesis by galectins involves the release of platelet-derived proangiogenic factors. PloS One 2014; 9(4): e96402.
7. Blood Components: American Red Cross; 2017:
Website
8. Treleaven J, Gennery A, Marsh J et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. British J. Haematol. 2011; 152(1): 35–51.
CrossRef
9. Tynngard N, Studer M, Lindahl TL, Trinks M, Berlin G. The effect of gamma irradiation on the quality of apheresis platelets during storage for 7 days. Transfusion 2008; 48(8): 1669–75.
CrossRef
10. Mason KD, Carpinelli MR, Fletcher JI et al. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128(6): 1173–86.
CrossRef
11. Kaufman RM, Djulbegovic B, Gernsheimer T et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann. Intern. Med. 2015; 162(3): 205–13.
12. Walther-Wenke G, Schmidt M. Impact of Bacterial Contamination on Blood Supply. Transfus. Med. Hemother. 2011; 38(4): 229–30.
13. Bacterial Contamination of Platelets: Centers for Disease Control and Prevention; 2013:
Website
14. Whitaker BI, Schlump K, Schulman J, Green J. Report of the US Department of Health and Human Services. The 2009 national blood collection and utilization survey report. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health. 2011.
15. Estcourt L. Why has demand for platelet components increased? A review. Transfus. Med. 2014; 24(5): 260–8.
16. Astori G, Amati E, Bambi F et al. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res. Ther. 2016; 7(1): 93.
17. Burnouf T, Strunk D, Koh MB, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016; 76: 371–87.
18. Tian BM, Wu RX, Bi CS, He XT, Yin Y, Chen FM. Human platelet lysate supports the formation of robust human periodontal ligament cell sheets. J. Tissue Eng. Regen. Med. 2017; doi:10.1002/term.2511.
CrossRef
19. Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J. Cell Physiol. 2007; 213(1): 18–26.
CrossRef
20. Lucarelli E, Fini M, Beccheroni A et al. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin. Orthop. Relat. Res. 2005; 435: 62–8.
21. Naaijkens BA, Niessen HW, Prins HJ et al. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012; 348(1): 119–30.
CrossRef
22. El Backly R, Ulivi V, Tonachini L, Cancedda R, Descalzi F, Mastrogiacomo M. Platelet lysate induces in vitro wound healing of human keratinocytes associated with a strong proinflammatory response. Tissue Eng. Part A. 2011; 17(13–14): 1787–800.
23. Chao Wang WS, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Biomed. Eng. 2017; 1: 1–10.
24. Shi Q, Montgomery RR. Platelets as delivery systems for disease treatments. Adv. Drug Deliv. Rev. 2010; 62(12): 1196–203.
25. Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J. Cosmet. Laser Ther. 2003; 5(1): 25–34.
CrossRef
26. Xu P, Zuo H, Chen B et al. Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci. Rep. 2017; 7: 42632.
27. Li J, Sharkey CC, Wun B, Liesveld JL, King MR. Genetic engineering of platelets to neutralize circulating tumor cells. J. Control Rel. 2016; 228: 38–47.
CrossRef
28. Rauch C, Feifel E, Amann EM et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX 2011; 28(4): 305–16.
CrossRef
29. Platelet Biogenesis 2016: Website
30. Feys HDaHB. Assays for quality control of platelets for transfusion. Int. Soc. Blood Transfusion Sci. Series 2013; 8: 221–4.
31. Dumont LJ, Cancelas JA, Graminske S et al. In vitro and in vivo quality of leukoreduced apheresis platelets stored in a new platelet additive solution. Transfusion 2013; 53(5): 972–80.
CrossRef
32. Administration USFaD. Drug Applications and Current Good Manufacturing Practice (CGMP) Regulations 2014:
Website
33. Giancola R, Bonfini T, Iacone A. Cell therapy: cGMP facilities and manufacturing. Muscles Ligaments Tendons J. 2012; 2(3): 243–7.
34. Baghbaderani BA, Tian X, Neo BH et al. cGMP-Manufactured Human Induced Pluripotent Stem Cells Are Available for Pre-clinical and Clinical Applications. Stem Cell Rep. 2015; 5(4): 647–59.
CrossRef
35. Thon JN, Medvetz DA, Karlsson SM, Italiano JE Jr. Road blocks in making platelets for transfusion. J. Thromb. Haemost. 2015; 13 Suppl. 1: S55–62.
36. Ramos-Mejia V, Melen GJ, Sanchez L et al. Nodal/Activin signaling predicts human pluripotent stem cell lines prone to differentiate toward the hematopoietic lineage. Mol. Ther. 2010; 18(12): 2173–81.
37. Feraud O, Valogne Y, Melkus MW et al. Donor Dependent Variations in Hematopoietic Differentiation among Embryonic and Induced Pluripotent Stem Cell Lines. PloS One 2016; 11(3): e0149291.
38. Mahdi BM. A glow of HLA typing in organ transplantation. Clin. Transl. Med. 2013; 2(1): 6.
39. Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology American Society of Hematology Education Program. 2007: 172–8.
40. Press Release. Cellular Dynamics Manufactures cGMP HLA “Superdonor” Stem Cell Lines to Enable Cell Therapy With Genetic Matching. Feb. 9, 2015. Cellular Dynamics International, Inc:
Website
41. Brown ME, Rondon E, Rajesh D et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PloS One 2010; 5(6): e11373.
42. Chen G, Gulbranson DR, Hou Z et al. Chemically defined conditions for human iPSC derivation and culture. Nature Methods 2011; 8(5): 424–9.
CrossRef
43. Hu K, Yu J, Suknuntha K et al. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood 2011; 117(14): e109–19.
44. Mack AA, Kroboth S, Rajesh D, Wang WB. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PloS One 2011; 6(11): e27956.
45. Yu J, Chau KF, Vodyanik MA, Jiang J, Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. PloS One 2011; 6(3): e17557.
46. Yu J, Hu K, Smuga-Otto K et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009; 324(5928): 797–801.
CrossRef
47. Baghbaderani BA, Syama A, Sivapatham R et al. Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications. Stem Cell Rev. 2016; 12(4): 394–420.
CrossRef
48. Services USDoHaH. CMC Postapproval Manufacturing Changes for Specified Biological Products To Be Documented in Annual Reports Guidance for Industry (Draft Guidance). In: Administration FaD (Ed.), Silver Spring, MD2017.
49. Thon JN, Mazutis L, Wu S et al. Platelet bioreactor-on-a-chip. Blood 2014; 124(12): 1857–67.
CrossRef
50. Chen VC, Ye J, Shukla P et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015; 15(2): 365–75.
CrossRef
51. Kempf H, Andree B, Zweigerdt R. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv. Drug Deliv. Rev. 2016; 96: 18–30.
52. Chan HF, Ma S, Leong KW. Can microfluidics address biomanufacturing challenges in drug/gene/cell therapies? Regen. Biomater. 2016; 3(2): 87–98.
53. Duffy DC, McDonald JC, Schueller OJ, Whitesides GM. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998; 70(23): 4974–84.
54. Regehr KJ, Domenech M, Koepsel JT et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 2009; 9(15): 2132–9.
CrossRef
55. Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip. 2006; 6(12): 1484–6.
CrossRef
56. Becker H. It’s the economy. Lab Chip. 2009; 9(19): 2759–62.
CrossRef
57. Avanzi MP, Oluwadara OE, Cushing MM, Mitchell ML, Fischer S, Mitchell WB. A novel bioreactor and culture method drives high yields of platelets from stem cells. Transfusion 2016; 56(1): 170–8.
CrossRef
58. Serra M, Brito C, Sousa MF et al. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J. Biotechnol. 2010; 148(4): 208–15.
59. Xu S, Gavin J, Jiang R, Chen H. Bioreactor productivity and media cost comparison for different intensified cell culture processes. Biotechnol. Prog. 2017; 33(4): 867–78.
60. Pollock J, Ho SV, Farid SS. Fed-batch and perfusion culture processes: economic, environmental, and operational feasibility under uncertainty. Biotechnol. Bioeng. 2013; 110(1): 206–19.
CrossRef
61. Skripchenko A, Myrup A, Thompson-Montgomery D, Awatefe H, Moroff G, Wagner SJ. Periods without agitation diminish platelet mitochondrial function during storage. Transfusion 2010; 50(2): 390–9.
CrossRef
62. Dumont LJ, Gulliksson H, van der Meer PF et al. Interruption of agitation of platelet concentrates: a multicenter in vitro study by the BEST Collaborative on the effects of shipping platelets. Transfusion 2007; 47(9): 1666–73.
CrossRef
63. Gong X, Liu H, Ding X et al. Physiological pulsatile flow culture conditions to generate functional endothelium on a sulfated silk fibroin nanofibrous scaffold. Biomaterials 2014; 35(17): 4782–91.
CrossRef
64. Prabhakarpandian B, Shen MC, Pant K, Kiani MF. Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature. Microvasc. Res. 2011; 82(3): 210–20.
65. Thon JN, Dykstra BJ, Beaulieu LM. Platelet bioreactor: accelerated evolution of design and manufacture. Platelets 2017; 28(5): 472–7.
CrossRef
66. DiCarlo AL, Poncz M, Cassatt DR, Shah JR, Czarniecki CW, Maidment BW. Medical countermeasures for platelet regeneration after radiation exposure. Report of a workshop and guided discussion sponsored by the National Institute of Allergy and Infectious Diseases, Bethesda, MD, March 22–23, 2010. Radiation Res. 2011; 176(1): e0001-15.
67. Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc. Natl Acad. Sci. USA 2014; 111(30): E3053–61.
68. Yan M, Jurasz P. The role of platelets in the tumor microenvironment: From solid tumors to leukemia. Biochim. Biophys. Acta 2016; 1863(3): 392–400.
69. Xu P, Wang R, Wang X, Ouyang J. Recent advancements in erythrocytes, platelets, and albumin as delivery systems. Onco. Targets Ther. 2016; 9: 2873–84.
CrossRef
70. Pacheco LD, Berkowitz RL, Moise KJ Jr, Bussel JB, McFarland JG, Saade GR. Fetal and neonatal alloimmune thrombocytopenia: a management algorithm based on risk stratification. Obstet. Gynecol. 2011; 118(5): 1157–63.
CrossRef
71. Trial to Reduce Alloimmunization to Platelets Study G. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. New Engl. J. Med. 1997; 337(26): 1861–9.
72. van de Watering L, Hermans J, Witvliet M, Versteegh M, Brand A. HLA and RBC immunization after filtered and buffy coat-depleted blood transfusion in cardiac surgery: a randomized controlled trial. Transfusion 2003; 43(6): 765–71.
CrossRef
73. Services USDoHaH. The 2011 National Blood Collection and Utilization Report. In: DHHS, editor. Washington, DC2013.
74. Blood Collection and Transfusion Market: A Global Perspective. Marketing Research Bureau; 2014.
75. Services CfMaM. Hospital Outpatient Prospective Payment – Final Rule with Comment Period and CY2015 Payment Rates. 2015.
76. Services CfMaM. Hospital Outpatient Prospective Payment System (OPPS). Limited Data Sal Files. 2015.
AFFILIATIONS
Jonathan N Thon*1,2,3 & Sven M Karlsson3
1 Hematology Division, Department of Medicine, Brigham and Women’s Hospital, MA 02115, USA
2 Harvard Medical School, Boston, MA, 02115 USA
3 Platelet BioGenesis, Boston, MA, 02125 USA
*Corresponding author: drjthon@gmail.com
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.