Obstacles for rAAV Clinical Trials: a question of vector supply and demand or know-how
Cell Gene Therapy Insights 2017; 3(9), 755-768.
10.18609/cgti.2017.075
Recent, positive, gene therapy clinical studies, using recombinant adeno-associated virus (rAAV) vectors, indicate the potential of this therapeutic platform for treating monogenic and acquired diseases. Although these studies are auspicious, realizing the full potential of rAAV gene therapy will require improved access to economical and scalable sources of vector. The complexities of the virus vector system and the limitations of transient production platforms have driven up cost-of-goods and extended the turn-around time for process development and manufacturing of clinical grade rAAV. This manufacturing bottleneck is likely to worsen due to the recent growth of the field, putting pressure on companies to address their clinical production strategies. The production platforms currently in use for clinical rAAV are discussed and we propose that direct experience is critically important for vector production campaigns to succeed in a timely manner.
Over the years, producing large-scale quantities of recombinant adeno-associated virus (rAAV) vectors to support large animal non-clinical studies and early-phase clinical trials have been an ongoing challenge for academic researchers and small biotechnology companies. The difficulty in producing high titers of rAAV can be attributed to a number of contributing factors, including the complexity of AAV vectorology and vector analytics, limitations of existing production systems, and the need for specialized manufacturing facilities. Recently reported positive clinical study results (AveXis pivotal Phase 2 or 3 for spinal muscular atrophy type 1 [1] ; Spark Therapeutics Phase 3 study for Leber’s congenital amaurosis type 2 [2] ; Biomarin Phase 1/2 study for hemophilia A [3] ; Voyager Therapeutics Phase 1b study for Parkinson’s disease [4] ) and the rapidly expanding popularity of rAAV vectors has exacerbated the bottleneck of clinical-grade vector despite an increase in the number of contract development and manufacturing organizations (CDMOs) who offer cGMP for AAV vectors production services. While the manufacturing of rAAV production will likely follow a trajectory similar to other biologics like monoclonal antibodies, where technology improvements and the distribution of know-how eventually enabled lower cost of goods and easier drug commercialization, it is still early days for gene therapy. Therefore, rAAV gene therapy companies are currently under inordinate pressure to meet the demands for rAAV vectors to support their research and clinical development programs and remain competitive. These biotechnology companies have limited choices where either they must develop internal capabilities or commit a substantial portion of their financial resources toward out-sourced vector production, or heavily prioritize their development programs. In either case, the assumed risk is that the rAAV manufacturing process technology is acquirable.
COMPLEXITY OF RECOMBINANT AAV VECTOR MANUFACTURING
Recombinant virus vectors are complex biological reagents where subtle changes in conditions, for example molecular reagent batch differences or variable producer cell characteristics, may have profound effects on both the vector quality and yield. Much of what is known about the molecular biology involved in rAAV vector generation is extrapolated from decades of research on wild-type (wt) AAV, which are non-pathogenic dependoparvoviruses that require a helper virus usually from the mastadenovirus (e.g., human adenovirus C) or simplexvirus (e.g., human alphaherpesvirus 1) genera for replication [5–8] . Replication of AAV requires a number of genes to be expressed in a coordinated manner, including AAV non-structural (Rep proteins [9–11] and assembly activating protein (AAP) [12–14] ), structural capsid (VP) proteins, as well as a set of helper virus genes, in a cell capable of supporting infection. The adenovirus and herpesvirus genes that are necessary for efficient rAAV production have been identified [5–8] . Despite the extensive study of these helper genes, however, the function that these gene products provided in an AAV infection has not been fully determined. In addition, the cytostatic and cytopathic effects of Rep and the helper virus proteins preclude the establishment of cell cultures that continuously produce rAAV. Thus, vector production is both a transient and terminal process that requires an efficient method of introducing the necessary virus genes into the cells that is reliable, scalable and cGMP compliant. While a variety of strategies have been developed to introduce the AAV and helper genes into mammalian and insect cells, no rAAV production platform has been fully optimized or standardized and each requires substantial expertise.
BRIEF DESCRIPTIONS OF RECOMBINANT AAV PRODUCTION PLATFORMS
HEK 293 cells & transient transfection
The conventional rAAV production process utilizes human embryonic kidney (HEK)293 cell and transient transfection (HEK/TT) is the most common strategy for producing rAAV for research purposes, pre-clinical and early clinical studies [15–19] . The widespread use of the HEK/TT platform is largely due to the ease of designing the molecular constructs and introducing the plasmid DNA into HEK293 cells with common chemical transfection reagents (organic media, e.g., polyethyleneimine (PEI) or inorganic media , e.g., calcium phosphate) [20] . While the system is relatively efficient in terms of vector particles produced per cell, it has a higher cost of goods due to the expense of producing plasmid DNA (pDNA) as a GMP starting material. Also, overall productivity is restricted by the plasmid transfection efficiency, since there is no cell-to-cell spread of pDNA. Only transfected cells are competent to produce rAAV limiting vector production to primary transfected cells. The recent development of HEK293 cell lines optimized for transfection in suspension culture has improved the scale of production [17,21] , but the scalability is still currently limited because of the low ratio of filled (transgene containing) particles that are typically removed to reduce the immunogenicity and particle aggregation, as well as to increase the potency of the drug product [25,26] . When the percentage of empty particles is low, generally ≤50% of the total particles, ion-exchange chromatographic methods may be used to ‘enrich’ for filled particles (albeit with appreciable recovery losses). The HEK/TT system, however, generates a large proportion of empty capsids, typically ≥80% of the total particles, which necessitates separation based on physical properties, such as buoyant density in cesium chloride or iodixanol ultracentrifugation gradients. Thus, the logistical and technical limitations are generally incompatible for commercial scale production volume or quantities, for example, single batch yields exceeding 4×1016 vector particles.
Spodopterafrugiperda cells &baculovirus expression vectors
A process using recombinant baculovirus expression vectors (BEVs) and invertebrate cell line derived from Spodopterafrugiperda (Sf9), referred to as Bac/Sf9, meets the definition for scalable production in serum-free culture medium [27] . Generally, a two or three BEV system is employed to deliver the AAV rep and cap genes along with the ITR-flanked transgene into suspension adapted Sf9 cells. Relatively small volume inocula, or low multiplicity of infection (MOI) of the BEVs are needed, since both the BEVs and rAAV are produced in the same cell line. The resulting cell-to-cell spread of the BEVs results in Sf9 cells simultaneously infected with the BEVs required to produce rAAV [28–31] . When the system is properly ‘tuned’, mostly full particles are produced (typically ≥70% of the total particles) and the biological activity of vectors generated in Sf9 is equivalent to HEK293 cell produced rAAV. However, non-optimized Sf9-based production systems can generate vector with decreased expression and incorporation of VP1 into the capsid relative to VP2, resulting in diminished biological activity. However, despite reports previously attributing the low activity of vectors to the platform, the activity improved with optimized Cap protein expression [32] .
Mammalian cells & recombinant herpes simplex virus
The herpes simplex virus (HSV) production platform uses recombinant herpes simplex virus type 1 (rHSV) based shuttle vectors to deliver the AAV rep and cap genes along with the ITR-flanked transgene into cells [33–38] . The rHSV system has been shown to have high specific productivity and can generate high quality rAAV vectors [36] . A benefit of the platform is that the rHSV shuttle vectors provide helper virus functions for rAAV production, thus alleviating an independent source of helper virus functions. In addition, rHSV shuttle vectors tolerate AAV Rep proteins that typically inhibit heterologous virus gene expression and replication [39–41] . Despite the theoretical elegance of the process, the major deficiency of the HSV platform is that the rHSV shuttle vectors and rAAV vectors are produced in different cell lines: the replication defective rHSV shuttle vectors are produced in complementary cell lines, usually V27 cells (Vero cells stably transformed with the HSV-1 UL54 gene encoding ICP27), whereas rAAV is produced in non-complementary cells, which do not support cell-to-cell spread of the rHSV vector. However, despite the complexities involved in the process, the resulting rAAV has been reported to have higher potency than vectors produced by TT/HEK. The enhanced activity partially compensates for the additional cost-of-goods of the starting materials [35] . Assuming that ≥1 pfu or rHSV per cell is optimal, each liter of culture would require ≥1×109 pfu. Therefore, a 100L rAAV production run involves producing ≥1 x1011 of high quality, replication-incompetent rHSV as a GMP starting material involving substantial effort, time, expertise and at a high cost. Several cell lines have been used for production of rAAV with rHSV vectors, including baby hamster kidney cells (BHK21) and HEK293 cells [38] . The system has been demonstrated to be amenable to both adherent and suspension cultures. Recently, a suspension adapted HEK293 cell line has been shown to generate vectors with very high titers using modified media and infection conditions, but production process scalability has not been reported using large-volume bioreactors [35–37] .
Mammalian producer cell lines & recombinant adenovirus infection
Producer cell lines have also been developed for rAAV production, usually by stably integrating the AAV rep and cap gene with the ITR-flanked transgene within a single cassette into HeLa, A549, or even HEK293 cells [42,43] . Once the producer cell lines have been generated and screened, the upstream process is relatively straightforward and production at 2000 liter has been demonstrated [44] . The process utilizes recombinant, replication competent, adenovirus (Ad) to stimulate rAAV production. Since adenovirus is capable of cell-to-cell spread and each cell bears the transgene and AAV rep/cap gene, all infectible cells potentially contribute to rAAV production. Stringent evaluation of the drug product is required to ensure removal of residual adenovirus contaminants. The process has not been widely employed, possibly due to the expertise and time required to establish, and optimize each packaging cell lines and then generating and characterizing the master cell bank for each new product.
Brief description of downstream manufacturing processes
Traditional downstream processing schemes for rAAV vectors have mostly relied on recovering intracellular particles by cell disruption using either freeze-thaw cycles, mechanical processes, for example sonication and homogenization, or chemical treatment, for example surfactants and non-ionic detergents. AAV vector particles are physically distinct from cellular constituent macromolecular assemblage, which enables separation using capsid-generic processes based on density ultracentrifugation through gradients of CsCl or iodixanol, or sedimentation rates through sucrose gradients [45–47] .
Polyethylene glycol (PEG) can also be added to the cell supernatant or clarified cell lysate to selectively precipitate the vector with low-speed centrifugation, although this method does not discriminate between empty and full particles. This process, which substantially reduces the feedstream biomass, is better suited for rAAV vector serotypes that are predominantly in the supernatant, rather than recovered from cell lysate [48–50] .
Affinity chromatography media based on heparin [51–53] or Cellufine™ sulfate [54] have proven useful for AAV serotypes that bind heparin, but have little utility for other serotypes. Ion exchange, either cation [55] or anion [56–60] has been used to capture rAAV particles from the crude cell lysates, but optimizing conditions are challenging resulting in a trade-off between recovery and purity. Thus, ion exchange chromatography is better suited as an orthogonal polish rather than initial capture step.
Scalable downstream purification of rAAV vectors has dramatically improved over the last few years with the development of recombinant immune affinity ligands derived from single-domain antibodies (sdAb) that are a natural component of the camelidae for example llama, immune repertoire. The epitope recognition domain or ‘paratope’ is within the heavy chain variable region (VHH) region that when expressed in microbial system, for example yeast or Escherichia coli, as an approximately 12kDa VHH ‘nanobody’ that can be covalently conjugated to activated chromatography media, for example NHS-Sepaharose (GE LifeSciences) for scalable axial flow (column) chromatography. AVB Sepharose (GE LifeSciences) is a commercially available immune affinity chromatography medium that was developed by screening a naïve llama phage display library for interactions with AAV1 capsids, but also interacts efficiently with several other natural AAV serotypes, including AAV2, AAV3, AAV5, AAV6, AAVrh10 and to a lesser degree AAV8 [61,62] . The POROS CaptureSelect affinity media (ThermoFisher) have also been developed to specifically bind AAV8- and AAV9-based vectors, providing a scalable capture step for these broadly used serotypes. A high degree of purity and recovery is achieved by the new affinity ligand columns, due to the specific affinity for the AAV capsids and good dynamic binding capacity, or avidity, such that host cell protein, host cell DNA and other process impurities are effectively eliminated with a single chromatographic process [63] . Ideally, an affinity resin can be developed that is truly pan-tropic; the new AAVX (ThermoFisher) has sought to achieve this and is reported to bind AAV serotypes 1-9 and other recombinants [64] , but it has not been fully vetted at this point. An alternative approach would be to develop the recently identified AAV common receptor (AAVR) for use as a universal ligand [65] .
The percentage of full rAAV vectors produced by transient transfection of HEK 293 cells ranges between 10 and 20% [26] requiring additional steps to enrich for full particles. This physical separation process, typically based on either buoyant density using isopycnic gradients or sedimentation rates using velocity centrifugation, limits the scalability and GMP-suitability of rAAV production [66] . Although several studies have demonstrated the feasibility of ion exchange chromatography to separate empty and full rAAV vector particles based on the slight differences of the exposed surface charges of the particles [67–69] , these studies are more reliable on analytical scales using high resolution, small diameter HPLC columns.
ANALYTICAL CHALLENGES
Assessing the quality of rAAV particles involve both physicochemical and biological assays. The vector is non-replicating and non-lytic, which increase the complexity of the biological activity assays. In addition, the physical properties of the particle limits the available techniques for characterizing rAAV. All serotypes of AAV are approximately 24 nm in diameter and are among the smallest viruses, yet relative to other biologics, for example monoclonal antibodies or subunit vaccines, the vector particle are large and complex macromolecular assemblies of protein and nucleic acid. The small size of AAV precludes the use of analytical procedures that are appropriate for inactivated virus vaccines or larger viral vectors, such as nanoparticle tracking analysis (direct observation and measurement of diffusion events using dynamic light scattering and Brownian motion) or by measuring changes in electrical resistance in a Coulter-type counter. AAV particles are also considerably larger than most other biologics, so techniques such as aggregation analysis by size exclusion chromatography are not practical and non-informative.
rAAV can be formulated at relatively high titers, for example ≥1014 vg (vector genome or full) particles per ml, which corresponds to ≥1 mg per ml of protein. However, direct analysis of the packaged DNA is often impractical because there is a single vector genome per particle and most direct nucleic acid assays utilize destructive process that consume large quantities of vector. Therefore, vector genome characterization rely primarily on PCR amplification to increase the detection sensitivity and reduce the consumption of vector. Quantitative PCR (qPCR) or digital droplet PCR (ddPCR) [70,71] are commonly employed to determine the vector genome concentration and to detect the presence and relative encapsidation of known DNA elements, for example plasmid-derived DNA. It has also been demonstrated, however, that the titers of DNase-resistant particles can vary dramatically from lab to lab, as was revealed in the evaluation of the AAV2 and AAV8 reference standards [72,73] . The use of different instruments and slight protocol or reagent changes possibly have a role, but much of the variability is likely due to inconsistencies in sample handling. Small errors or variations in sampling technique, or dispensing the reagents, etc., are amplified during PCR, thus, it is imperative that titering by qPCR or ddPCR is performed in a laboratory with experience in using these methods for quantifying viral vectors and qualifying the assays for measuring materials for clinical use.
Currently, the most convincing technique for determining the empty to full capsid ratio is based on analytical ultracentrifugation. Another popular method uses two tools: qPCR quantification of vector genomes (full particles) and ELISA quantification of total capsid (full plus empty particles). The standard error obtained with one tool may exceed the value obtained with the other tool, for example qPCR genome concentration = 2.3 x 1012 per ml ± 1 x 1012, ELISA measurement of total capsids = 3 x 1012 ± 0.5E x 1012. Full-particles obtained by isopycnic gradient is a useful reagent to calibrate the qPCR and ELISA results. Another approach to evaluate the empty:full ratio based on negative-staining transmission electron microscopy is unreliable due to idiosyncrasies of heavy metal uptake into particles leading to inaccurate full and empty particle determination.
The biological assays are critically important and are also the most difficult to establish, qualify as a reliable analytical assay. The vector potency is typically determined in a cell culture – based assay that relates gene expression to vector ‘dose’ based on quantitative readouts, for example ELISA or qPCR. Since rAAV vectors may be considered as a pro-drug or transgene delivery reagent, the potency of the vector must be determined through in vivo studies or cell-based assays in which the rAAV vector transduces the cell and the activity of the resulting protein, or RNA, or knockdown of a target protein, is measured. If the delivered gene expresses an enzyme or an siRNA, then the activity may be measured directly. However, rAAV vectors are being utilized to treat a variety of diseases in which the function of the expressed protein is structural or not well understood; in these cases it is especially challenging to measure the effective strength of the vector for example Serca2a [74,75] or dystrophin [76–81] where protein expression levels may be surrogates for potency.
rAAV vectors, like all biologic medicines, must be evaluated to insure that the residual impurities from the host cell are at acceptably safe levels. In addition, it is imperative to identify and quantify process impurities resulting from the introduction of the AAV and helper genes into the producer cells. These concerns are amplified for production platforms that utilize viruses, especially when the viruses are potentially pathogenic. In these cases, it is critical that highly sensitive assays are developed to detect and measure any replication-competent viruses as well as their nucleic and protein components in the final product.
MARKET ANALYSIS
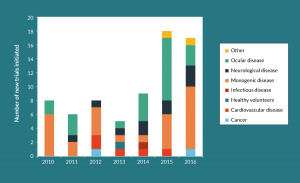
As gene therapy has started to show more clinical success over the past decade, there has been a clear shift from academic to industry-sponsored programs and clinical trials. The number of companies developing therapies based on rAAV vectors has dramatically increased in the past few years from a handful to more than 40, encompassing more than 130 programs. While about a quarter of these programs are being developed for treatments of the eye and will presumably use small doses of rAAV vector that are relatively easy to produce, the majority likely require high titers of the vector. A review of the available information on the number of clinical trials using rAAV vectors from 2010 through 2016 shows a sharp rise in the number of new trials from about 8 per year about 18, with a large percentage being for non-ocular disorders (Figure 1)[82] . Indeed, the dose of vector required for systemic administration to treat lysosomal storage diseases and the muscular dystrophies is approaching 1 x 1015 vg per patient. To produce these large quantities of vector, companies are faced with the question of build versus buy, invest in the process development, cGMP, and quality infrastructure or outsource the manufacturing to a CDMO.
CGMP INFRASTRUCTURE
Independent of the production platform employed, producing clinical-grade rAAV requires a facility that meets the regulatory requirements for containment, airflow and room air exchange, utilities including power, water, and lighting, accessibility, temperature, environmental monitoring, etc., as specified in the US Code of Federal Regulations (21 CFR § 210, 211, 610). Cleanroom facilities are generally constructed with cascades of pressure and air cleanliness, where the most important manufacturing steps (e.g., vial filling) are performed in the spaces with the highest air quality. Viral vector manufacturing cleanrooms are typically built with extra airflow controls to limit the risk of cross-contamination. In addition, a number of supporting mechanical systems are needed, such as clean water and steam generators, back-up power, temperature regulated drug substance/product storage, as well as facility and equipment monitoring systems. Other controlled spaces are needed for the storage of quarantined and released raw materials used in the manufacturing and testing of the cGMP vectors. An analytical laboratory adjacent to the clean rooms is convenient for assessing in-process and drug substance quality, as well as the testing of environmental monitoring samples to verify that the facility is meeting cleanliness standards. Also, access to a process development (PD) laboratory capable of generating rAAV vectors using a process and scale that is representative of the clinical-grade manufacturing platform, provides an economical alternative to using cleanrooms for non-cGMP activities and reduces the pressure for cleanroom scheduling.
In addition, sufficient quality oversight is required from a quality assurance (QA) unit that is independent of the production group. The QA group is responsible for ensuring that the manufacturing and testing of the vectors is performed in accordance with the regulations and in compliance with the FDA and International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines through establishing and maintaining a robust quality system. The quality system establishes clear processes for the review, approval and control of operation procedures, material control, internal and external quality audits, validation and calibration of utilities and process equipment; training and qualification of personnel, as well as managing and investigating any deviations and related events.
FILLING THE PRODUCTION GAP AT CONTRACT DEVELOPMENT MANUFACTURING ORGANIZATIONS
Very few companies with gene therapy programs have elected to invest in their own, internal manufacturing capabilities, further increasing the queue for CDMO services to produce cGMP-grade rAAV vectors. As a result, anticipating an increased demand for clinical trial material and large-scale commercial manufacturing, several CDMOs have recently either entered the market or expanded their operations. Due to this increase in cleanroom space as well as pre-existing space built over the last several decades for biologics, for example monoclonal antibodies or recombinant protein production, the bottleneck for cGMP-grade rAAV vector is not likely due to physical limitations of cleanrooms or bioreactors. Much less common are CDMOs who have successfully completed multiple production campaigns for cGMP vector. During the past 20+ years, many publications and presentations from academic researchers and biotechnology companies have reported advances and breakthroughs in rAAV vector production, yet with the exception of the processes described above, few of these methods have been developed into a robust, manufacturing platform consistent with cGMP best practices [33,73,83–91] . It is unlikely that CDMOs are experienced with each of these processes, and each client may have a preferred production platform and downstream process that has not been previously employed by the CDMO.
KNOW-HOW
It is unsurprising that establishing a de novorAAV vector manufacturing strategy and qualifying analytical methods present challenges for CDMOs, especially for contractors lacking familiarity or expertise with rAAV vectors. As such, inexperienced CDMOs, unaccustomed with the nuances of the protocols, are more likely to encounter production inconsistencies resulting from process deviations than more knowledgeable CDMOs. Since there are few CDMOs who have a track record of successfully completing multiple, large-scale production campaigns for cGMP rAAV vector, these providers tend to capitalize a greater market share and are able to command a premium for their know-how.
The inevitable spread of knowledge and increased experience with rAAV vector manufacturing at less utilized CDMOs will eventually level the playing field, lower costs through competition, and increase the availability of large-scale, cGMP-grade rAAV vectors. Until then companies are faced with the difficult decision of how to invest in manufacturing in order to stay competitive.
FINANCIAL & COMPETING INTERESTS DISCLOSURE
David J Dismuke and Robert M Kotin are inventors on patents related to recombinant AAV technology and own equity in gene therapy-related companies. To the extent that the work in this manuscript increases the value of these commercial holdings the authors have a conflict of interest. Portions of the technology described in this report are covered by United States and European patents assigned to the Secretary of the Department of Health and Human Services. A fraction of the licensing fees and royalty payments made to the NIH is distributed to the inventors (Robert M Kotin) in accordance with US Government and NIH policy. No writing assistance was utilized in the production of this manuscript.
REFERENCES
1. FDA Approves AveXis’ Pivotal Trial of AVXS-101 for SMA Type 1. Website
2. Spark Therapeutics Announces Publication in The Lancet of Pivotal Phase 3 Clinical Trial Data for Investigational VoretigeneNeparvovec. Website
3. BioMarin’s Investigational Gene Therapy for Hemophilia A Maintains Average Factor VIII Levels within Normal Range for over One Year. Website
4. Voyager Therapeutics Announces Positive Results from Ongoing Phase 1b Trial of VY-AADC01 for Advanced Parkinson’s Disease. Website
5. Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 1998; 9(18): 2745–60.
CrossRef
6. Stutika C, Huser D, Weger S, Rutz N, Hessler M, Heilbronn R. Definition of herpes simplex virus helper functions for the replication of adeno-associated virus type 5. J. Gen. Virol. 2015; 96(Pt 4): 840–50.
CrossRef
7. Weindler FW, Heilbronn R. A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J. Virol. 1991;65(5): 2476–83.
8. Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998; 72(3): 2224–32.
9. Hermonat PL, Labow MA, Wright R, Berns KI, Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J. .Virol. 1984; 51(2): 329–39.
10. Im DS, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell 1990; 61(3): 447–57.
CrossRef
11. Pereira DJ, McCarty DM, Muzyczka N. The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection. J. Virol. 1997; 71(2): 1079–88.
12. Earley LF, Powers JM, Adachi K et al. Adeno-associated Virus (AAV) Assembly-Activating Protein Is Not an Essential Requirement for Capsid Assembly of AAV Serotypes 4, 5, and 11. J. Virol. 2017; 91(3).
CrossRef
13. Sonntag F, Kother K, Schmidt K et al. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J. Virol. 2011; 85(23): 12686–97.
CrossRef
14. Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl Acad. Sci. USA 2010; 107(22): 10220–5.
CrossRef
15. Allay JA, Sleep S, Long S et al. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum. Gene Ther. 2011; 22(5): 595–604.
CrossRef
16. Clement N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016; 3: 16002.
CrossRef
17. Grieger JC, Soltys SM, Samulski RJ. Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol. Ther. 2016; 24(2): 287–97.
CrossRef
18. Lai CM, Estcourt MJ, Himbeck RP et al. Preclinical safety evaluation of subretinal AAV2.sFlt-1 in non-human primates. Gene Ther. 2012; 19(10): 999–1009.
CrossRef
19. Wright JF, Wellman J, High KA. Manufacturing and regulatory strategies for clinical AAV2-hRPE65. Curr. Gene Ther. 2010; 10(5): 341–9.
CrossRef
20. van der Loo JC, Wright JF. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet. 2016; 25(R1): R42–52.
21. Chahal PS, Schulze E, Tran R, Montes J, Kamen AA. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods 2014; 196: 163–73.
CrossRef
22. Gao K, Li M, Zhong L et al. Empty Virions In AAV8 Vector Preparations Reduce Transduction Efficiency And May Cause Total Viral Particle Dose-Limiting Side-Effects. Mol. Ther. Methods Clin. Dev. 2014; 1(9): 20139.
CrossRef
23. Nathwani AC, Rosales C, McIntosh J et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011; 19(5): 876–85.
CrossRef
24. Wright JF. AAV empty capsids: for better or for worse? Mol. Ther. 2014; 22(1): 1–2.
CrossRef
25. Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008; 15(11): 840–8.
CrossRef
26. Wright JF. Transient transfection methods for clinical adeno-associated viral vector production. Hum. Gene Ther. 2009; 20(7): 698–706.
CrossRef
27. Cecchini S, Virag T, Kotin RM. Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02- to 200-liter cultures. Hum. Gene Ther. 2011; 22(8): 1021–30.
CrossRef
28. Airenne KJ, Hu YC, Kost TA et al. Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol. Ther. 2013; 21(4): 739–49.
CrossRef
29. Kotin RM, Snyder RO. Manufacturing Clinical Grade Recombinant Adeno-Associated Virus Using Invertebrate Cell Lines. Hum. Gene Ther. 2017; 28(4): 350–60.
CrossRef
30. Negrete A, Kotin RM. Large-scale production of recombinant adeno-associated viral vectors. Methods Mol. Biol. 2008; 433: 79–96.
CrossRef
31. Virag T, Cecchini S, Kotin RM. Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum. Gene Ther. 2009; 20(8): 807–17.
CrossRef
32. Mietzsch M, Hering H, Hammer EM, Agbandje-McKenna M, Zolotukhin S, Heilbronn R. OneBac 2.0: Sf9 Cell Lines for Production of AAV1, AAV2, and AAV8 Vectors with Minimal Encapsidation of Foreign DNA. Hum. Gene Ther. Methods 2017; 28(1): 15–22.
CrossRef
33. Clement N, Knop DR, Byrne BJ. Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies. Hum. Gene Ther. 2009; 20(8): 796–806.
CrossRef
34. Wu Z, Wu X, Cao H, Dong X, Wang H, Hou Y. A novel and highly efficient production system for recombinant adeno-associated virus vector. Sci. China C Life Sci. 2002; 45(1): 96–104.
CrossRef
35. Kang W, Wang L, Harrell H et al. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009; 16(2): 229–39.
CrossRef
36. Adamson-Small L, Potter M, Falk DJ, Cleaver B, Byrne BJ, Clement N. A scalable method for the production of high-titer and high-quality adeno-associated type 9 vectors using the HSV platform. Mol. Ther. Methods Clin Dev. 2016; 3: 16031.
CrossRef
37. Knop DR, Harrell H. Bioreactor production of recombinant herpes simplex virus vectors. Biotechnol. Prog. 2007; 23(3): 715–21.
CrossRef
38. Thomas DL, Wang L, Niamke J et al. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene Ther. 2009; 20(8): 861–70.
CrossRef
39. Antoni BA, Rabson AB, Miller IL, Trempe JP, Chejanovsky N, Carter BJ. Adeno-associated virus Rep protein inhibits human immunodeficiency virus type 1 production in human cells. J. Virol. 1991; 65(1): 396–404.
40. Horer M, Weger S, Butz K, Hoppe-Seyler F, Geisen C, Kleinschmidt JA. Mutational analysis of adeno-associated virus Rep protein-mediated inhibition of heterologous and homologous promoters. J. Virol. 1995; 69(9): 5485–96.
41. Needham PG, Casper JM, Kalman-Maltese V, Verrill K, Dignam JD, Trempe JP. Adeno-associated virus rep protein-mediated inhibition of transcription of the adenovirus major late promoter in vitro. J. Virol. 2006; 80(13): 6207–17.
CrossRef
42. Chadeuf G, Favre D, Tessier J et al. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirus-induced amplification of the integrated rep-cap genome. J. Gene Med. 2000; 2(4): 260–8.
<a href=”https://doi.org/10.1002/1521-2254(200007/08)2:43.0.CO;2-8 ” rel=”noopener” target=”_blank”> CrossRef
43. Clark KR, Voulgaropoulou F, Johnson PR. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 1996; 3(12): 1124–32.
44. Thorne B, Takeya R, Vitelli F, Swanson X. Gene Therapy. Adv. Biochem. Eng. Biotechnol. 2017 Mar; 14.
45. Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006; 1(3): 1412–28.
CrossRef
46. Guo P, Xiao X, El-Gohary Y et al. A simplified purification method for AAV variant by polyethylene glycol aqueous two-phase partitioning. Bioengineered. 2013; 4(2): 103–6.
CrossRef
47. Lock M, Alvira M, Vandenberghe LH et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010; 21(10): 1259–71.
CrossRef
48. Arden E, Metzger JM. Inexpensive, serotype-independent protocol for native and bioengineered recombinant adeno-associated virus purification. J. Biol. Methods 2016; 3(2).
49. Guo P, El-Gohary Y, Prasadan K et al. Rapid and simplified purification of recombinant adeno-associated virus. J. Virol. Methods 2012; 183(2): 139–46.
CrossRef
50. Piras BA, Tian Y, Xu Y, Thomas NA, O’Connor DM, French BA. Systemic injection of AAV9 carrying a periostin promoter targets gene expression to a myofibroblast-like lineage in mouse hearts after reperfused myocardial infarction. Gene Ther. 2016; 23(5): 469–78.
CrossRef
51. Auricchio A, Hildinger M, O’Connor E, Gao GP, Wilson JM. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 2001; 12(1): 71–6.
CrossRef
52. Clark KR, Liu X, McGrath JP, Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 1999; 10(6): 1031–9.
CrossRef
53. Zolotukhin S, Byrne BJ, Mason E et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999; 6(6): 973–85.
CrossRef
54. O’Riordan CR, Lachapelle AL, Vincent KA, Wadsworth SC. Scaleable chromatographic purification process for recombinant adeno-associated virus (rAAV). J. Gene Med. 2000; 2(6): 444–54.
<a href=” https://doi.org/10.1002/1521-2254(200011/12)2:63.0.CO;2-1″ rel=”noopener” target=”_blank”> CrossRef
55. Debelak D, Fisher J, Iuliano S, Sesholtz D, Sloane DL, Atkinson EM. Cation-exchange high-performance liquid chromatography of recombinant adeno-associated virus type 2. J. Chromatogr. B Biomed. Sci. Appl. 2000; 740(2): 195–202.
CrossRef
56. Lock M, Alvira MR, Wilson JM. Analysis of particle content of recombinant adeno-associated virus serotype 8 vectors by ion-exchange chromatography. Hum. Gene Ther. Methods 2012; 23(1): 56–64.
CrossRef
57. Smith RH, Yang L, Kotin RM. Chromatography-based purification of adeno-associated virus. Methods Mol. Biol. 2008; 434: 37–54.
CrossRef
58. Brument N, Morenweiser R, Blouin V et al. A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol. Ther. 2002; 6(5): 678–86.
CrossRef
59. Gao G, Qu G, Burnham MS et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. . 2000; 11(15): 2079–91.
CrossRef
60. Kaludov N, Handelman B, Chiorini JA. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Hum. Gene Ther. 2002; 13(10): 1235–43.
CrossRef
61. Smith RH, Levy JR, Kotin RM. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009; 17(11): 1888–96.
CrossRef
62. Wang Q, Lock M, Prongay AJ, Alvira MR, Petkov B, Wilson JM. Identification of an adeno-associated virus binding epitope for AVB sepharose affinity resin. Mol. Ther. Methods Clin. Dev. 2015; 2: 15040.
CrossRef
63. Ye GJ, Scotti MM, Thomas DL, Wang L, Knop DR, Chulay JD. Herpes Simplex Virus Clearance during Purification of a Recombinant Adeno-associated Virus Serotype 1 Vector. Hum. Gene Ther. Clin. Dev. 2014; Oct 01.
64. PorosCaptureSelect AAV Resins: AAV8, AAV9, AAVX User Guide., ThermoScientific Pub. No. 100038399, Rev. D, 13 August 2017: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/100038399_POROS_CapSel_AAV8_AAV9_Resins_UG.pdf
65. Pillay S, Meyer NL, Puschnik AS et al. An essential receptor for adeno-associated virus infection. Nature 2016; 530(7588): 108–12.
CrossRef
66. Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. Methods Clin. Dev. 2016; 3: 15049.
CrossRef
67. Davidoff AM, Ng CY, Sleep S et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Methods 2004; 121(2): 209–15.
CrossRef
68. Qu G, Bahr-Davidson J, Prado J et al. Separation of adeno-associated virus type 2 empty particles from genome containing vectors by anion-exchange column chromatography. J. Virol. Methods 2007; 140(1–2): 183–92.
CrossRef
69. Urabe M, Nakakura T, Xin KQ et al. Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J. Virol. 2006; 80(4): 1874–85.
CrossRef
70. Lock M, Alvira MR, Chen SJ, Wilson JM. Absolute determination of single-stranded and self-complementary adeno-associated viral vector genome titers by droplet digital PCR. Hum. Gene Ther. Methods 2014; 25(2): 115–25.
CrossRef
71. Werling NJ, Satkunanathan S, Thorpe R, Zhao Y. Systematic Comparison and Validation of Quantitative Real-Time PCR Methods for the Quantitation of Adeno-Associated Viral Products. Hum. Gene Ther. Methods 2015; 26(3): 82–92.
CrossRef
72. D’Costa S, Blouin V, Broucque F et al. Practical utilization of recombinant AAV vector reference standards: focus on vector genomes titration by free ITR qPCR. Mol. Ther. Methods Clin. Dev. 2016; 5: 16019.
CrossRef
73. Lock M, McGorray S, Auricchio A et al. Characterization of a recombinant adeno-associated virus type 2 Reference Standard Material. Hum. Gene Ther. 2010; 21(10): 1273–85.
CrossRef
74. Goonasekera SA, Lam CK, Millay DP et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J. Clin. Invest. 2011; 121(3): 1044–52.
CrossRef
75. Shin JH, Bostick B, Yue Y, Hajjar R, Duan D. SERCA2a gene transfer improves electrocardiographic performance in aged mdx mice. J. Transl. Med. 2011; 9: 132.
CrossRef
76. Bish LT, Sleeper MM, Forbes SC et al. Long-term restoration of cardiac dystrophin expression in golden retriever muscular dystrophy following rAAV6-mediated exon skipping. Mol. Ther. 2012; 20(3): 580–9.
CrossRef
77. Bostick B, Shin JH, Yue Y, Wasala NB, Lai Y, Duan D. AAV micro-dystrophin gene therapy alleviates stress-induced cardiac death but not myocardial fibrosis in >21-m-old mdx mice, an end-stage model of Duchenne muscular dystrophy cardiomyopathy. J. Mol. Cell Cardiol. 2012; 53(2): 217–22.
CrossRef
78. Gardner KL, Kearney JA, Edwards JD, Rafael-Fortney JA. Restoration of all dystrophin protein interactions by functional domains in trans does not rescue dystrophy. Gene Ther. 2006; 13(9): 744–51.
CrossRef
79. Koo T, Okada T, Athanasopoulos T, Foster H, Takeda S, Dickson G. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. J. Gene Med. 2011; 13(9): 497–506.
CrossRef
80. Wang B, Li J, Fu FH, Xiao X. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J. Orthop. Res. 2009; 27(4): 421–6.
CrossRef
81. Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation 2003; 108(13): 1626–32.
CrossRef
82. Clinical Trials database: https://clinicaltrials.gov
83. Clark KR, Voulgaropoulou F, Fraley DM, Johnson PR. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995; 6(10): 1329–41.
CrossRef
84. Conway JE, Rhys CM, Zolotukhin I et al. High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap. Gene Ther. 1999; 6(6): 986–93.
CrossRef
85. Drittanti L, Jenny C, Poulard K et al. Optimised helper virus-free production of high-quality adeno-associated virus vectors. J. Gene Med. 2001; 3(1): 59–71.
<a href=” https://doi.org/10.1002/1521-2254(2000)9999:99993.0.CO;2-U” rel=”noopener” target=”_blank”> CrossRef
86. Gao GP, Qu G, Faust LZ et al. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum. Gene Ther. 1998; 9(16): 2353–62.
CrossRef
87. Okada T, Nomoto T, Yoshioka T et al. Large-scale production of recombinant viruses by use of a large culture vessel with active gassing. Hum. Gene Ther. 2005; 16(10): 1212–8.
CrossRef
88. Qu G, McClelland A, Wright JF. Scaling-up production of recombinant AAV vectors for clinical applications. Curr. Opin. Drug Discov. Devel. 2000; 3(6): 750–5.
89. Schmeer M, Schleef M. Pharmaceutical grade large-scale plasmid DNA manufacturing process. Methods Mol. Biol. 2014; 1143: 219–40.
CrossRef
90. Tamayose K, Hirai Y, Shimada T. A new strategy for large-scale preparation of high-titer recombinant adeno-associated virus vectors by using packaging cell lines and sulfonated cellulose column chromatography. Hum. Gene Ther. 1996; 7(4): 507–13.
CrossRef
91. Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002; 13(16): 1935–43.
CrossRef
AFFILIATIONS
David J Dismuke1& Robert M Kotin2
1 StrideBio, Inc., Durham, NC, USA.
d.dismuke@stridebio.com
2 University of Massachusetts Medical School, Gene Therapy Center, Worcester, MA, USA.
robert.kotin@umassmed.edu

This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.