Automated, spinning membrane filtration for preparation of mobilized leukapheresis products for CD34+ cell selection
Cell Gene Therapy Insights 2017; 3(8), 623-637.
10.18609/cgti.2017.059
CD34+ hematopoietic stem and progenitor cells are used to promote bone marrow reconstitution following cancer treatment and may offer a novel treatment for other indications. Leukapheresis of mobilized peripheral blood (mPB) is a common source of CD34+ cells. Depending on the application, it may be desired to purify the CD34+ cells in the leukapheresis product via immunomagnetic selection. Prior to selection, the leukapheresis product must be washed to remove platelets and unbound paramagnetic beads. When performed manually, these processing steps are time-consuming and operator intensive. This study evaluated the LOVO Cell Processing System (LOVO), a commercially available instrument utilizing spinning membrane filtration, as an automated alternative for preparing mPB leukapheresis products for CD34+ cell selection. The LOVO removed >90% of platelets prior to bead incubation and substantially reduced pre-selection processing time compared to a manual approach. Products prepared using the LOVO had an average 76.2 ± 2.9 % CD34+ cell recovery and 4.72 ± 0.41 log T cell reduction following selection.
CD34+ hematopoietic stem and progenitor cells (HSPCs) are often used to promote bone marrow reconstitution following ablative therapy for certain cancers. Due to concerns over T-cell-dependent graft-versus-host disease [1] and B-cell mediated Epstein–Barr virus-related lymphoproliferative disorders following allogeneic transplant [2], as well as the risk of malignant cells in autologous leukapheresis products [3], CD34+ cells may be purified prior to infusion. CD34+ cells have also been evaluated as novel cell therapy treatments for sickle cell disease [4], β-thalassemia [5], critical limb ischemia [6] and HIV [7], among others. Manufacturing of these therapies commonly involves immunomagnetic selection of CD34+ cells as an initial processing step.
HSPCs may be collected from granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood through an apheresis procedure [8]. Following the leukapheresis collection, the CliniMACS Plus instrument (Miltenyi) may be used for automated selection of CD34+ cells [9]. Prior to processing on the CliniMACS Plus, the leukapheresis product must be prepared for selection. This process involves platelet (PLT) reduction and suspension in a selection buffer, followed by incubation with 50-nm, super-paramagnetic iron-dextran beads coated with CD34 antibody. After incubation, a wash is performed to remove excess, unbound beads. Automated processing on the CliniMACS Plus passes the cells over a column in the presence of a magnetic field, resulting in the retention of bead-labeled, CD34+ cells and the flow-through of unlabeled cells. The purified CD34+ cell fraction is then eluted from the column. More recently, the CliniMACS Prodigy (Prodigy; Miltenyi) has become available for automated, closed-system, combined preparation (washes, reagent incubation) and CD34+ cell selection of mPB leukapheresis products. The Prodigy’s integrated centrifuge chamber is used to perform wash steps.
The manual preparation process for CliniMACS Plus selection involves repeated centrifugation, supernatant depletion, wash buffer addition, and cell resuspension steps, as well as many welding and sealing steps to connect and remove waste and buffer bags. These steps require significant operator interaction and careful monitoring (i.e., to ensure white cells are not removed during supernatant removal with a plasma extractor) and are time-consuming. Spohn et al. estimated that approximately 4 hours are required for manual preparation of a leukapheresis product for CliniMACS Plus selection (estimate excludes CliniMACS Plus instrument setup and operation time) [10]. In an effort to reduce hands-on interaction and improve robustness, several groups have explored alternative workflows for pre-selection processing. Three centers in a multi-center study of CD34+ cell-enriched, T-cell-depleted grafts for acute myeloid leukemia used the Cobe 2991 cell washer (Terumo) [9], while Zinno et al., Scerpa et al., and Tran et al. used the Cytomate (Baxter) [11], Sepax S-100 (Biosafe) [12] and Elutra (Terumo) [13], respectively.
Several studies have observed an inverse correlation between pre-selection PLT content and post-selection CD34+ cell recovery and/or purity of the CD34+ cell fraction. Three manual centrifugation-based washes prior to CD34 reagent addition resulted in >95% PLT removal and 81.8% CD34+ cell recovery post selection, compared to ~24% PLT removal and 71.2% CD34+ cell recovery with a single wash [14]. Tran et al. saw higher PLT removal and purity of the post-selection CD34+ cell fraction when elutriation, instead of three manual centrifugation washes, was performed [13]. Stroncek et al. saw a statistically significant higher PLT content in the CD34-negative fraction of cells prepared and selected using the CliniMACS Prodigy, compared to those that were centrifuged and selected using the CliniMACS Plus [15]. The higher PLT content observed with the CliniMACS Prodigy corresponded with lower CD34+ cell recovery (50.1%), compared to the CliniMACS Plus (66%). Sites that used the Cobe 2991 cell washer, as opposed to manual centrifugation washes, reported higher CD34+ cell recovery (72.6 vs 63%) in a multi-center study [9]. Data from one center showed that Cobe 2991 processing resulted in ~65% PLT removal, leading the authors to postulate that higher PLT removal, compared to published studies with manual centrifugation prior to selection, may have been responsible for the higher CD34+ cell recovery.
The LOVO Cell Processing System (LOVO; Fresenius Kabi) is designed for automated white cell processing and supports closed-system processing through the use of a sterilized, single-use, disposable kit. Unlike centrifugation-based devices, the LOVO uses spinning membrane filtration to separate cells and supernatant. Filtration occurs in the LOVO kit’s spinning membrane module (spinner), which consists of an outer housing and an inner rotor wrapped with a 4-µm-pore, polycarbonate membrane. As a suspension flows through the space between the spinner’s outer housing and inner rotor, supernatant and cells 4 µm are retained and exit through a separate, retentate port (Figure 1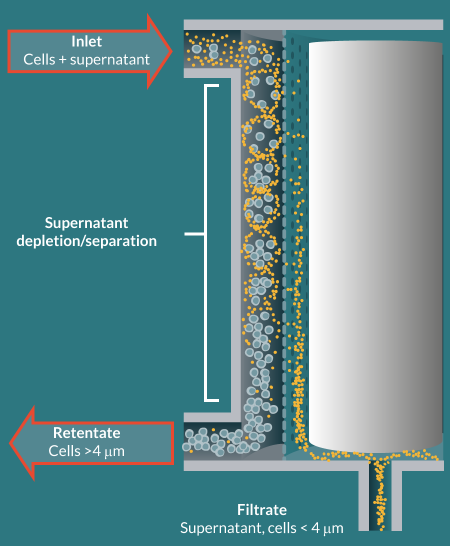
Motivated by studies suggesting that higher PLT removal from leukapheresis products results in better post-selection CD34+ cell recovery and CD34+ cell fraction purity, as well as data showing the LOVO system’s ability to deplete PLTs [17], this study evaluated the LOVO for automating the washing steps involved in the preparation of mPB leukapheresis products for CliniMACS Plus CD34+ cell selection.
Methods
Leukapheresis procedures were performed on G-CSF-mobilized, healthy donors using either the COBE Spectra Apheresis System (Terumo) or Spectra Optia Apheresis System CMNC (Terumo). Leukapheresis products were shipped overnight from the collection site to the manufacturing site in an insulated shipper (1–10°C; NanoCool). Prior to processing, the leukapheresis products were brought to room temperature and transferred from the original collection bag to a 600-mL transfer pack through a 150-µm filter. Filtration is not required prior to LOVO processing, but was performed to match a previously established protocol. The filtered product was sampled immediately prior to processing on the LOVO and the time of sampling was used to calculate the elapsed time between the end of the leukapheresis collection and the start of processing. An XP-300 hematology analyzer (Sysmex) was used to determine white blood cell (WBC) and PLT concentrations, as well as hematocrit (HCT) %, which was calculated as “Hemoglobin (Hb) x 3” [18]. Flow cytometry was performed using a FACSCanto (BD), using ISHAGE gating, with fluorescent antibodies against CD45, CD34 and CD3 antigens, as well as a 7-AAD viability dye. Absolute CD34+ cell counts were calculated as “WBC concentration (Sysmex) x % CD34+ in CD45+ population (FACSCanto)”. Absolute CD3+ cell counts were calculated as “WBC concentration (Sysmex) x % CD3+ in CD45+ population (FACSCanto)”.
Two LOVO protocols were developed and saved to the LOVO instrument (version 2.0 software) for immediate access at the time of leukapheresis processing. The LOVO Wash 1 protocol was designed to remove PLTs and plasma and resuspend cells in CliniMACS PBS/EDTA Buffer (Miltenyi) supplemented with 0.5% HSA (PBS/EDTA/HSA buffer). Key procedure parameters for the LOVO Wash 1 protocol are shown in Table 1. LOVO procedure setup involved operator entry of the leukapheresis product volume and WBC concentration, HCT % and PLT concentration, as well as the desired final product volume. Based on this information, the instrument displayed an estimated volume of wash solution (PBS/EDTA/HSA buffer) required for the procedure.
| Table 1: Integral protocol design parameters for LOVO Wash 1 and Wash 2. | ||
|---|---|---|
| LOVO Wash 1 | LOVO Wash 2 | |
| Wash cycles | 2 | 2 |
| Spinner inlet flow rate | 80 mL/min | 150 mL/min |
| Reduction retentate flow rate | 8 mL/min | 30 mL/min |
| Desired spinner inlet PCV | 0.06 | 0.06 |
| Reduction spinner revolution rate | 4000 rpm | 3000 rpm |
As CliniMACS PBS/EDTA buffer is available in 1-L bags, 1 L of PBS/EDTA/HSA buffer was prepared for convenience, despite the fact that 1 L was often substantially more buffer than the estimated required volume. The LOVO Cell Processing Disposable Kit (Fresenius Kabi) was installed on to the instrument, and the standard retentate (final product) bag was replaced with a 600-mL transfer pack via sterile tubing welding. A spike transfer set was used to add a tubing lead to a 1-L PBS/EDTA/HSA buffer bag for connection to the LOVO kit via sterile tubing welding. If more than one bag of wash solution was prepared, a Y-type connector set with spikes was used to connect two bags. The PBS/EDTA/HSA buffer and leukapheresis product were attached to the LOVO kit via sterile tubing welding, and the operator began the LOVO procedure. The LOVO protocol was configured with mid-procedure automated pauses to allow the operator to mix the leukapheresis and LOVO kit In-Process bags to enhance rinse steps or mix cells with wash solution. At the end of LOVO Wash 1, the LOVO screen displayed a weight-based assessment of the final product volume and the total automated procedure time, which were recorded by the operator. This time represented the duration of all automated portions of the procedure, including kit check and wash buffer prime prior to the start of cell processing. The final product bag was sealed off from the LOVO kit, transferred to a biosafety cabinet, and sampled for hematology analyzer counts and flow cytometry analysis. One vial of CliniMACS CD34 Reagent (Miltenyi) was then injected into the product. The cells were incubated with the reagent for 30 minutes at room temperature on an orbital shaker.
During the incubation period, the kit from the LOVO Wash 1 procedure was removed from the instrument. The LOVO Wash 2 procedure, which was designed to remove unbound reagent (50-nm paramagnetic beads) and resuspend cells in fresh PBS/EDTA/HSA buffer, was set up using post-LOVO-Wash-1 measured WBC and PLT concentrations and HCT %. Key procedure parameters for the LOVO Wash 2 protocol are shown in Table 1. A new LOVO kit was installed and the standard retentate bag was replaced with a 600-mL transfer pack. One liter of PBS/EDTA/HSA buffer was attached, except in one run where the estimated wash buffer was 952 mL and 1200 mL (~25% excess) was attached. As soon as the reagent incubation was complete, the labeled product was attached to the LOVO kit via sterile welding. Automated pauses were also used in the LOVO Wash 2 procedure. At the end of LOVO Wash 2, the final product volume and total automated procedure time were recorded from the LOVO display. The final product bag was sealed off from the LOVO kit and sampled for hematology analyzer counts.
For CD34+ cell selection, the LOVO Wash 2 final product bag was attached to a CliniMACS Tubing Set LS (Miltenyi) via the standard pre-system filter (Pall) in a biosafety cabinet. The LS tubing set was chosen based on a study showing higher CD34+ cell recovery, compared to the TS tubing set [19]. The tubing set was installed on a CliniMACS Plus Instrument (Miltenyi) and Program 2 was initiated. Following selection, the cell collection bag (positive fraction) was sampled for hematology analyzer counts and flow cytometry analysis.
Statistical analysis
Results are expressed as mean ± 1 standard deviation (SD).
Data & results
An average of 17.6 ± 0.6 hours elapsed between the end of the leukapheresis collection and the start of LOVO processing. Collected products had an average volume of 318 ± 37 mL and contained an average of 44.2 ± 12.4 x 109 WBCs, 1.8 ± 0.6 % HCT (5.6 ± 1.4 mL of RBCs), 697 ± 234 x 109 PLTs, 277 ± 110 x 106 CD34+ cells, and 15.8 ± 7.3 x 109 CD3+ cells (Table 2).
| Table 2: Leukapheresis products collected from G-CSF-stimulated healthy donors. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Run | Apheresis device and program | Volume (mL) | WBC (x109) | HCT (%) | RBC (mL) | PLT (x109) | Packed cell volume (PCV, %) | CD34+ cells (x109) | CD3+ cells (x109) |
| 1 | Optia CMNC | 297 | 58.5 | 2.4 | 7.1 | 959 | 12.8 | 287 | 22.8 |
| 2 | Spectra MNC | 361 | 37.9 | 1.2 | 4.3 | 623 | 6.8 | 163 | 16.3 |
| 3 | Optia CMNC | 296 | 36.1 | 1.8 | 5.3 | 509 | 8 | 383 | 8.2 |
| Mean | – | 318 | 44.2 | 1.8 | 5.6 | 697 | 9.2 | 277 | 15.8 |
| SD | – | 37 | 12.4 | 0.6 | 1.4 | 234 | 3.2 | 110 | 7.3 |
During setup for each LOVO procedure, the WBC and PLT concentrations, as well as HCT %, were entered into the system by the operator. Using assumed cell volumes of 400 fL and 8 fL for a WBC and PLT, respectively, the LOVO calculated the Packed Cell Volume (PCV) % for the WBC and PLT components, then summed these percentages with the HCT % to calculate a total PCV % for each leukapheresis product. The leukapheresis PCV % was used to determine the amount of AutoDilution to be performed during the LOVO procedure. AutoDilution is the automated addition of wash solution to the cell suspension just upstream of the spinner inlet in order to dilute the cell suspension to the maximum spinner inlet PCV % parameter, which is an integral setting in the LOVO protocol. AutoDilution avoids the overloading of cells in the spinner, thereby avoiding fouling of the membrane, and allows a single LOVO procedure to easily adapt to changes in the starting leukapheresis material cell content. The amount of wash solution to be used during AutoDilution was included in the total estimated wash solution required for the procedure that was displayed to the operator during LOVO procedure setup.
The LOVO Wash 1 protocol was designed to deplete PLTs and plasma from the starting leukapheresis product and resuspend the cells in selection buffer at a smaller, specified volume in preparation for reagent incubation. LOVO Wash 1 data is shown in Table 3. In Run 1 and 2 of the LOVO Wash 1 procedures, a 95-mL final product volume was targeted, whereas 105 mL was targeted in Run 3 to accommodate for a planned increased sampling volume. Other than in Run 2, where the LOVO produced a final product volume 3 mL larger than the targeted final product volume, the LOVO generated the final product volume specified during procedure setup. LOVO Wash 1 automated processing, the sum of all automated steps from the kit check through to the end of the procedure, took an average of 24.1 ± 1.9 minutes. WBC recovery and CD34+ cell recovery averaged 91.2 ± 5.7% and 90.9 ± 1.2%, respectively, and PLT reduction averaged 92 ± 3.4%, resulting in 53 ± 19 x 109 PLTs remaining in the product prior to incubation with CD34 Reagent. An average of 642 ± 184 mL of PBS/EDTA/HSA buffer was required for the procedure.
| Table 3: LOVO Wash 1 data. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Run | Estimated wash solution required (mL) | Target final product volume (mL) | Actual final product volume (mL) | Automated processing time (min) | WBC recovery (%) | CD34+ cell recovery (%) | PLT depletion | PLT content (x 109) |
| 1 | 902 | 95 | 98 | 26.7 | 84.6 | 89.8 | 95.2 | 46 |
| 2 | 494 | 95 | 95 | 23.2 | 90.5 | 92.6 | 87.3 | 79 |
| 3 | 531 | 105 | 105 | 22.4 | 98.6 | 90.2 | 93.6 | 33 |
| Mean | 642 | 98 | 99 | 24.1 | 91.2 | 90.9 | 92 | 53 |
| SD | 184 | 5 | 4 | 1.9 | 5.7 | 1.2 | 3.4 | 19 |
LOVO Wash 2 data is shown in Table 4. For LOVO Wash 2, the calculated PCV % for each leukapheresis product was higher than for LOVO Wash 1, owing to the smaller leukapheresis product volumes and thereby higher cell concentrations. 824 ± 90 mL of PBS/EDTA/HSA buffer was required for the procedure. In all three runs, the actual final product volume matched the targeted final product volume of 275 mL. Average automated processing time was 17.6 ± 0.9 minutes and average WBC recovery and PLT depletion were 101.1 ± 6.2% and 96.4 ± 3.9%, respectively. The >100% WBC recovery in Run 1 can likely be attributed to a non-representative sample taken after LOVO Wash 1, where WBC recovery was calculated to be only 84.6%. Flow cytometry was not performed on the Post-LOVO-Wash-2 product, therefore CD34+ cell recovery for Wash 2 alone could not be calculated. Cumulative WBC recovery and PLT depletion through both LOVO Wash 1 and Wash 2 procedures averaged 91.9 ± 2.2% and 99.8 ± 0.0%, respectively.
| Table 4: LOVO Wash 2 data. | |||||||
|---|---|---|---|---|---|---|---|
| Run | Packed cell volume (PCV, %) | Estimated wash solution required (mL) | Target final product volume (mL) | Actual final product volume (mL) | Automated processing time (min) | WBC recovery (%) | PLT depletion (%) |
| 1 | 26.6 | 952 | 275 | 275 | 18.9 | 109.7 | 90.9 |
| 2 | 19.9 | 757 | 275 | 275 | 16.9 | 98.8 | 98.3 |
| 3 | 18.6 | 764 | 275 | 275 | 17.2 | 94.9 | 100 |
| Mean | 21.7 | 824 | 275 | 275 | 17.6 | 101.1 | 96.4 |
| SD | 3.5 | 90 | 0 | 0 | 0.9 | 6.2 | 3.9 |
| The post-Wash 2 PLT count for Run 2 was below the hematology analyzer’s limit of detection and was therefore assumed to be 0, resulting in a calculated PLT depletion of 100%. | |||||||
The data for the CliniMACS Plus selection of CD34+ cells is shown in Table 5. CD34+ cell viability and purity averaged 99.8 ± 0.1% and 93 ± 1.0%, respectively. Flow cytometry was not performed on the Post-LOVO-Wash-2 product, therefore recovery of CD34+ cells sent to the CliniMACS Plus was not calculated. Cumulative CD34+ cell recovery, calculated as the number of CD34+ cells in the CliniMACS Plus positive fraction divided by the number of CD34+ cells in the unmanipulated leukapheresis product, averaged 76.2 ± 2.9%. CD3+ cells comprised on average 0.13% of the CliniMACS Plus positive fraction and average cumulative CD3+ depletion measured 4.72 ± 0.41 logs.
| Table 5: CliniMACS Plus positive fraction data. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Run | Volume (mL) | CD34+ cell viability (%) | CD34+cell fraction purity (%) | CD34+ cells (x106) | Cumulative CD34+ cell recovery (%) | CD3+ purity (%) | CD3+ cells (x109 | Cumulative log depletion of CD3+ cells |
| 1 | 87.7 | 99.8 | 92.5 | 207 | 72.3 | 0.12 | 0.26 | 4.94 |
| 2 | 82.8 | 99.8 | 92.2 | 125 | 76.9 | 0.13 | 0.17 | 4.98 |
| 3 | 85 | 99.9 | 94.4 | 304 | 79.4 | 0.15 | 0.47 | 4.24 |
| Mean | 85.2 | 99.8 | 93 | 212 | 76.2 | 0.13 | 0.3 | 4.72 |
| SD | 2 | 0.1 | 1 | 73 | 2.9 | 0.01 | 0.13 | 0.41 |
Discussion
The LOVO Cell Processing System uses spinning membrane filtration to remove supernatant and cells or particles <4 µm in size from mixed cell suspensions, such as leukapheresis products. This study evaluated the LOVO for performing the wash steps that occur prior to CD34+ cell immunomagnetic selection of mPB leukapheresis products from healthy donors. After overnight shipment, each product was processed using the LOVO Wash 1 protocol to remove PLTs and suspend cells in PBS/EDTA/HSA buffer at the appropriate volume for labeling. The washed product was removed from the LOVO, incubated with anti-CD34 paramagnetic beads, then processed using the LOVO Wash 2 protocol to remove excess, unbound beads and suspend cells in PBS/EDTA/HSA buffer at the appropriate volume for selection on the CliniMACS Plus. The results are summarized in Figure 2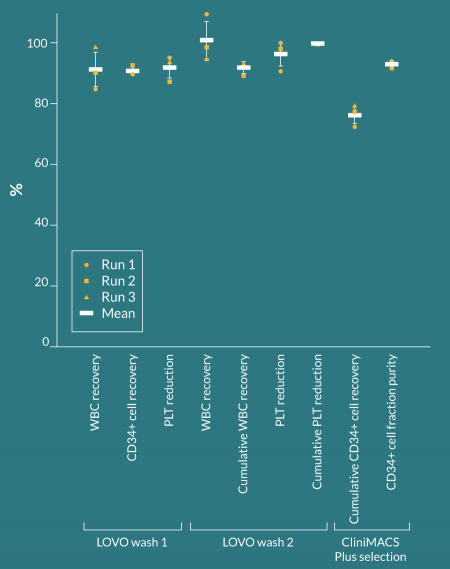
During each LOVO procedure setup, the operator enters the desired final product volume. The total packed cell volume (PCV) of each starting leukapheresis product, calculated from the operator-entered WBC, RBC, and PLT concentrations, determines the minimum final product volume that can be produced. In Wash 1 Run 1, the actual minimum achievable final product volume was 98 mL, 3 mL higher than the operator-entered desired final product volume of 95 mL. In all runs, RBC content in the leukapheresis product was determined by a hematology analyzer. Due to the analyzer’s linear range of hemoglobin (Hb) extending to lower values than the linear range of HCT %, HCT % was calculated as “Hb x 3” [18] rather than using the HCT % readout from the analyzer. However, hematology analyzers can report falsely high hemoglobin levels for samples with high (> 5 x 104/µL) WBC concentrations [20]. For comparison, in this study the average WBC concentration in the unmanipulated mPB leukapheresis product was 14.1 ± 0.5 x 104/µL. Hematology analyzers also overestimate HCT % in products with high WBC concentrations, particularly when the HCT % is low (0.5–3%) [21]. When the RBC content is inflated, the Packed Cell Volume (PCV) calculated by the LOVO is also inflated, meaning that more wash buffer than necessary will be used to dilute the cell suspension prior to entering the spinning membrane module (AutoDilution). This, in turn, increases the minimum achievable final product volume for the LOVO procedure.
Since completing this study, additional LOVO runs were performed where the HCT % of the leukapheresis was determined by centrifuging a sample of the product in a capillary tube, then reading the HCT % with a microhematocrit reader disk [22]. The microhematocrit method typically resulted in a smaller HCT % compared to a hematology analyzer measurement for the same sample (data not shown). LOVO runs performed after entry of a microhematocrit-based HCT %, rather than a hematology-analyzer based HCT %, for RBC content have shown similar WBC recovery and PLT removal to that observed in this study [Data not shown]. Use of the HCT % from the microhematocrit method decreases the calculated PCV of the leukapheresis product, which in turn decreases the calculated LOVO minimum achievable final product volume. In addition, the microhematocrit method avoids processing an undiluted leukapheresis sample on a hematology analyzer, which may be required to ensure that the RBC concentration will be above the analyzer’s lower limit of detection, but which can also clog the analyzer’s aperture.
The average automated processing times for Wash 1 and 2 were 24.1 ± 1.9 and 17.6 ± 0.9 minutes, respectively. Four automated pauses were built into each LOVO protocol to allow for mixing of bags to enhance rinse steps or mix cells with wash solution. Each pause requires <30 seconds of operator interaction with the instrument, meaning that the total processing time is increased by ~2 minutes when pauses are accounted for. Before beginning each procedure, the operator enters information about the starting leukapheresis product (volume and cell concentrations) as well as the targeted final product volume. The operator then modifies (if desired) and installs the LOVO disposable kit. The LOVO kit includes a standard, 800-mL final product bag, but because reagent incubation is typically performed in a 600-mL transfer pack, that bag was used to replace the standard final product bag in this study. The LOVO captures the empty weight of all installed bags, meaning that the final product volume displayed at the end of the procedure is accurate, even if a standard bag had been replaced. Finally, the wash solution and leukapheresis are attached. Conservative time estimates for these steps are shown in Table 6. In total, including a 45-minute estimate for reagent addition and incubation, the pre-selection process utilizing the LOVO requires ~2 hours, which is 2 hours less than the manual process [10,23]. The CliniMACS Plus operates for ~45 minutes to 1 hour [10,23], with prior setup of tubing and priming adding ~30 minutes [10]. If the CliniMACS Plus setup begins after the LOVO Wash 2 ends, the total processing time through to the end of CliniMACS plus selection is ~3.5 hours. If CliniMACS Plus setup can be performed ahead of time, the total processing time could be reduced to ~3 hours. In comparison, the Prodigy procedure for CD34+ cell selection, including tubing set installation, is ~5 hours [10,23].
| Table 6: Estimated total processing time for pre-selection preparation using the LOVO for all washing steps. | ||
|---|---|---|
| Time (min) | ||
| LOVO wash 1 | LOVO wash 2 | |
| Procedure setup (information entry) | 3 | 3 |
| Kit modification (optional) and installation | 10 | 10 |
| Wash solution and leukapheresis product attachment | 5 | 5 |
| Automated processing | 24 | 18 |
| Mid-procedure automated pauses | 2 | 2 |
| Total | 44 | 38 |
| Combined LOVO Wash 1 and Wash 2 Total | 82 | |
| Combined LOVO total + 45 min for reagent addition and incubation | 127 | |
Several studies have demonstrated that reduction of the PLT content in the leukapheresis product during pre-processing steps results in improved CD34+ cell immunomagnetic selection. Stroncek et al. measured PLTs in the CliniMACS Plus and Prodigy negative fractions as a way to approximate the PLT content prior to CD34+ cell election on each instrument. The Prodigy had higher PLT content (207.1 ± 44.5 x 109) and lower recovery (51.4 ± 8.2%) compared to the CliniMACS Plus (91.6 ± 58.1 x 109, 65.1 ± 15.7%). It is likely that the PLT content at the time of reagent addition was higher than the amount measured in the negative fraction, for both selection approaches, because additional wash steps were performed following reagent incubation. PLTs are thought to interfere with binding of the paramagnetic-bead-bound antibody to target cells [11], in turn reducing the affinity of target cells for the magnetized column. The LOVO’s 4-µm pore membrane is capable of removing PLTs (2–3 µm) while retaining larger WBCs (>9 µm [24]). In this study, an average of 92 ± 3.4 % of PLTs were removed during LOVO Wash 1, resulting in an average of 53 ± 19 x 109 PLTs in the product prior to reagent addition. This PLT content is less than the negative fraction PLT content for both the CliniMACS Plus and Prodigy in the study by Stroncek et al. It is also important to note that the mPB leukapheresis products processed in this study were shipped to the manufacturing site at refrigerated temperatures. PLTs stored cold have been observed to spontaneously aggregate upon warming [25], but if this phenomenon occurred, it did not affect the LOVO’s ability to filter out platelets.
There are many published studies on CD34+ cell immunomagnetic selection of mPB leukapheresis products from healthy donors. Various groups have investigated different methods for performing the pre-processing wash steps prior to selection on the CliniMACS. More recently, groups have compared manual pre-processing and CliniMACS selection with the Prodigy’s combined, automated preparation and selection. Figure 3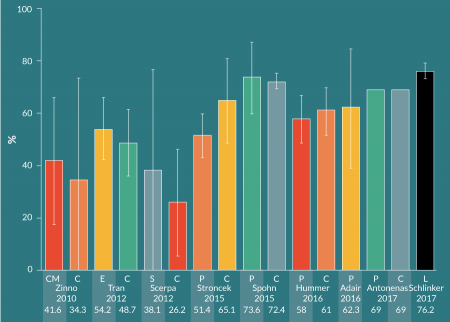
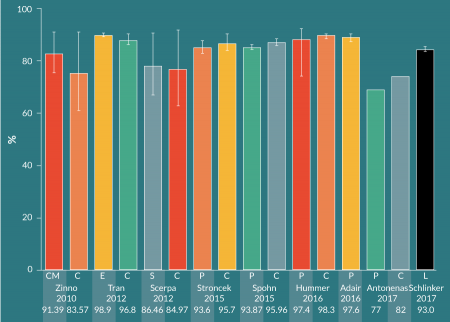
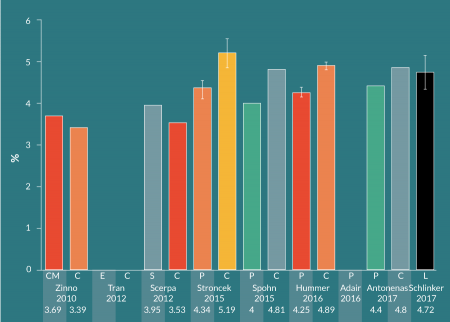
For mPB leukapheresis products containing ≤ 0.6 x 109 CD34+ cells out of a total WBC population of ≤ 60 x 109, the manufacturer recommends to use the CliniMACS TS tubing set. The LS (large-scale) tubing set is to be used with products containing >0.6 x 109 but ≤ 1.2 x 109 CD34+ cells out of > 60 x 109 but ≤ 120 x 109 WBCs. Schumm et al. found that the LS tubing set resulted in higher CD34+ cell recovery (median: 80%, range: 45-120%), compared to the TS tubing set (median: 72%, range: 27–130%) [19]. The LS tubing set was used in this study, despite the fact that each mPB leukapheresis product contained <0.6 x 109 CD34+ cells and <60 x 109 WBCs. Cumulative CD34+ cell recovery in the current study with LOVO pre-processing was the highest among the sample of CliniMACS studies shown here (Figure 5), and may be partially due to the use of the LS tubing set. Except for Hummer et al, who used the TS tubing set [26], the other studies in the sample did not provide tubing set information. Importantly, Schumm et al. did not observe significant differences in CD34+ cell fraction purity and cell recovery, nor T cell depletion, between the TS and LS tubing sets.
Conclusion
Selection of CD34+ cells may be performed to avoid complications with allogeneic stem cell transplants in standard-of-care treatments, and can also provide a starting population for new HSPC-based cell therapies. The CliniMACS Plus is often used to perform immunomagnetic selection of CD34+ cells from mPB leukapheresis products, which must undergo several washing steps in preparation for selection. These steps are performed to achieve PLT reduction, suspension in selection buffer and reduction of excess, unbound beads following reagent incubation. Several groups have evaluated options for automating these steps, but some of these options are no longer available or offer only semi-automated solutions. When performed manually with a centrifuge, plasma extractor and weigh scale, these pre-processing steps may take 4 hours [10,23].
This study provides a preliminary, n = 3 investigation of the use of the LOVO Cell Processing System for preparing mPB leukapheresis products from healthy donors for CD34+ cell immunomagnetic selection using the CliniMACS Plus. Priority was placed on processing entire mPB leukapheresis products, rather than splitting the available products to perform technical replicates, in order to assess the LOVO instrument’s ability to process relevant cell numbers and determine the total time required. A larger study, ideally one where mPB leukapheresis products could be halved and processed on either the LOVO, then selected using the CliniMACS Plus or processed entirely on the CliniMACS Prodigy, would provide a useful comparison of the two available approaches for pre-selection preparation and CD34+ cell selection. However, given the fairly consistent results, this small study demonstrated applicability of the LOVO for this process. Futhermore, the CD34+ cell recovery, CD34+ purity, and T-cell depletion from this study were shown to be similar or better than those reported by comparable studies (Figures 3–5), including those that evaluated other automated technologies.
Use of the LOVO offers several benefits for preparing leukapheresis products for immunomagnetic selection. Unlike manual centrifugation, the LOVO process requires little operator interaction beyond setup and occasional bag manipulation and reduces total pre-selection processing time by several hours over the manual centrifugation approach. The total setup and processing time for the LOVO steps, followed by CliniMACS Plus selection, is also estimated to be ~1.5–2 hours less than that required for the Prodigy approach. With respect to product quality, the LOVO efficiently removes PLTs, reducing the total PLT content prior to reagent incubation beyond the amount that has been correlated with lower post-selection CD34+ recovery.
Disclaimer
The LOVO Cell Processing system is for laboratory use only. Unless the user has obtained advance clearance or approval from the appropriate regulatory agency, cells processed on this system are not intended for diagnostic purposes, direct transfusion, or for use in the production of therapeutic products or vaccines for clinical use. For applications requiring regulatory clearance or approval, users may request the required LOVO technical documentation from Fresenius Kabi to support their submissions.
Financial & Competing interests disclosure
Alaina C Schlinker is an employee of Fresenius Kabi USA, LLC. No writing assistance was utilized in the production of this manuscript.
References
1. Kawabata Y, Hirokawa M, Komatsuda A, Sawada K. Clinical applications of CD34+ cell-selected peripheral blood stem cells. Ther. Apher. Dial. 2003: 7; 298–304.
CrossRef
2. Comoli P, Basso S, Labirio M, Baldanti F, Maccario R, Locatelli F. T cell therapy of Epstein–Barr virus and adenovirus infections after hemopoietic stem cell transplant. Blood Cells. Mol. Dis. 2008; 40: 68–70.
CrossRef
3. Vogel W, Scheding S, Kanz L, Brugger W. Clinical applications of CD34(+) peripheral blood progenitor cells (PBPC). Stem Cells 2000; 18: 87–92.
CrossRef
4. Ribeil J-A, Hacein-Bey-Abina S, Payen E et al. Gene Therapy in a Patient with Sickle Cell Disease. N. Engl. J. Med. 2017; 376: 848–855.
CrossRef
5. Canver MC, Orkin SH. Customizing the genome as therapy for the -hemoglobinopathies. Blood 2016; 127: 2536 LP-2545.
6. Losordo DW, Kibbe MR, Mendelsohn F et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ. Cardiovasc. Interv. 2012; 5: 821–30.
CrossRef
7. Kiem H-P, Jerome KR, Deeks SG, McCune JM. Hematopoietic stem cell-based gene therapy for HIV disease. Cell Stem Cell. 2012; 10: 137–47.
CrossRef
8. Bendall LJ, Bradstock KF. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014; 25: 355–67.
CrossRef
9. Keever-Taylor CA, Devine SM, Soiffer RJ et al. Characteristics of CliniMACS® System CD34-Enriched T Cell-Depleted Grafts in a Multicenter Trial for Acute Myeloid Leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Protocol 0303. Biol. Blood Marrow Transplant. 2012; 18: 690–7.
CrossRef
10. Spohn G, Wiercinska E, Karpova D et al. Automated CD34+ cell isolation of peripheral blood stem cell apheresis product. Cytotherapy 2015; 17(10): 1465–71.
CrossRef
11. Zinno F, Landi F, Aureli V et al. Positive immunomagnetic CD34(+) cell selection in haplo-identical transplants in beta-thalassemia patients: removal of platelets using an automated system. Cytotherapy 2010; 12; 60–6.
CrossRef
12. Scerpa MC, Daniele N, Ciammetti C et al. Cell processing for haplo-identical hematopoietic stem cell transplantation: automated washing and immunomagnetic-positive selection. Cytotherapy 2012; 14: 811–7.
CrossRef
13. Tran CA, Torres-Coronado M, Gardner A et al. Optimized processing of growth factor mobilized peripheral blood CD34+ products by counterflow centrifugal elutriation. Stem Cells Transl. Med. 2012; 1: 422–9.
CrossRef
14. Del Fante C, Perotti C, Viarengo G et al. Immunomagnetic cell selection performed for HLA haploidentical transplants with the CliniMACS device: effect of additional platelet removal on CD34+ cell recovery. Stem Cells Dev. 2005; 14: 734–9.
CrossRef
15. Stroncek DF, Tran N, Frodigh SE et al. Preliminary evaluation of a highly automated instrument for the selection of CD34+ cells from mobilized peripheral blood stem cell concentrates. Transfusion 2016; 56: 511–7.
CrossRef
16. Noris P, Klersy C, Zecca M et al. Platelet size distinguishes between inherited macrothrombocytopenias and immune thrombocytopenia. J. Thromb. Haemost. 2009; 7: 2131–6.
CrossRef
17. Wegener C, Heber C, Min K. Novel cell washing device using spinning membrane filtration. Cytotherapy 2013; 15: S27.
CrossRef
18. Bain B, Bates I. Basic haematological techniques. In: Lewis S, Bain B, Bates I (Eds). Pract. Haematol. 9th Ed., Churchill Livingston, Edinburgh, 2001: 19–46.
19. Schumm J, Lang P, Kuci S, Greil J, Niethammer D. Positive enrichment of CD34+ cells with the CliniMACS device: Effect of column sizes on separation results. Bone Marrow Transplant. 2002; 29: S200.
20. Sarma PR, Red Cell Indices. In: Walker HK, Hall WD, Hurst JW (Eds.). Boston, 1990.
21. Avecilla ST, Marionneaux SM, Leiva TD et al. Comparison of manual hematocrit determinations versus automated methods for hematopoietic progenitor cell apheresis products. Transfusion 2016; 56: 528–32.
CrossRef
22. Billett HH. Hemoglobin and Hematocrit. In: Walker HK, Hall WD, Hurst JW (Eds.). Boston, 1990.
23. Antonenas V, Yehson K, Tong D et al. Preliminary validation study of the new CliniMACS Prodigy for the selection of CD34+ cells from mobilized peripheral blood stem cell products. Cytotherapy 2017; 19: S49.
CrossRef
24. Hümmer C, Poppe C, Bunos M et al., Automation of cellular therapy product manufacturing: results of a split validation comparing CD34 selection of peripheral blood stem cell apheresis product with a semi-manual vs. an automatic procedure. J. Transl. Med. 2016; 14: 76.
CrossRef
25. Brown RI. The physics of continuous flow centrifugal cell separation. Artif. Organs. 1989; 13: 4–20.
CrossRef
26. Kattlove HE, Alexander B. The Effect of Cold on Platelets. I. Cold-induced Platelet Aggregation. Blood 1971; 38; 39 LP-48.
27. Adair JE, Waters T, Haworth KG et al. Semi-automated closed system manufacturing of lentivirus gene-modified haematopoietic stem cells for gene therapy. Nat. Commun. 2016; 7: 13173.
CrossRef
Affiliation
Alaina C Schlinker
Manager, Cell Therapy Application Support, Fresenius Kabi, USA

This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.