Emerging use of cord blood in regenerative medicine
Cell Gene Therapy Insights 2017; 3(7), 573-581.
10.18609/cgti.2017.058
Regenerative medicine is dedicated to the study of repairing, replacing or regenerating damaged human cells, tissues or organs to restore or establish normal function; and it has potential applications to treat a wide variety of conditions. Umbilical cord blood (CB) is a relatively safe, easily collected, readily available and non-controversial source of cells for regenerative medicine purposes. If it proves to be useful in this regard, there will be significant implications in both the treatment of diseases and the current models of CB collection.
The field of regenerative medicine is dedicated to the study of repairing, replacing or regenerating damaged human cells, tissues or organs to restore or establish normal function [1]. The ultimate goal, of course, is to develop methods to treat previously untreatable conditions and improve outcomes of injuries and diseases for which traditional therapies are inadequate. Regenerative approaches including novel medical devices, tissue engineering and generation of artificial organs, and cellular therapies are all currently in development and/or under investigation across a wide range of disciplines.
Investigations in cellular therapy – utilizing cells therapeutically to not just replace lost or damaged cells, but to affect/change existing cells and ongoing disease processes – have increased rapidly and have numerous potential applications. Sources of cells for such purposes vary extensively, each with their own associated potential risks and benefits. This article will focus on the use of umbilical cord blood (CB) as a source of cells for emerging cellular therapies, summarize the scope of ongoing clinical trials, and highlight their potential applications in neurologic conditions.
Umbilical cord blood as a source of cells for regenerative therapies
As a source of cells for developing therapeutic cell products, CB has several unique qualities. CB is an abundantly available source of cells that can be harvested at no risk to the mother or infant donor. It is routinely collected, cryopreserved and banked, making it readily available and accessible on demand. Many infectious agents are much less common in newborns and donating mothers undergo extensive donor screening, making CB a relatively safe source of cells with a low risk of transmitting infections. Importantly, CB is also a non-controversial source of cells as it has historically been discarded as medical waste after birth along with the placenta.
Umbilical CB is a well-established source of cells for hematopoietic stem cell transplantation (HSCT), in which donor CB cells are used to replace the recipient’s hematopoietic and immune systems. Compared to bone marrow, CB is more readily available, requires less stringent HLA-matching, has a lower incidence of graft-versus-host disease, and is less likely to transmit infections via latent viruses. As such, CB has made HSCT available for many patients lacking a suitable bone marrow donor and has become the preferred donor source in certain clinical scenarios. In over 30 years of use in allogeneic, unrelated HSCT, CB has not been shown to cause any teratomas or solid tumors [2].
After HSCT, donor CB cells engraft in the bone marrow and distribute throughout the body. In fact, CB donor-derived tissue-specific cells have been identified in multiple organs in both animals and humans after HSCT, including the liver [3], lung, pancreas [3,4], skeletal muscle [5] and brain [6], indicating that transplanted CB cells are capable of repopulating more than just the hematopoietic system [7,8]. This may be due to the presence of a true embryonic-like stem cell in CB or small numbers of committed but tissue-specific, non-hematopoietic progenitors. Nonetheless, while CB cells have the ability to differentiate into tissue-specific cells and integrate into host organs, there is growing evidence that their therapeutic effects in settings other than traditional HSCT, may result more from their ability to initiate tissue repair by activating host cells via paracrine effects.
Scope of ongoing studies
The relative ease of collection, processing, testing and storage of CB along with its potential therapeutic properties, make it an attractive source of cells for regenerative medicine applications across many disciplines. In fact, a recent systematic review identified 57 published clinical studies involving a total of 814 patients that utilized CB as cell therapy for novel indications in regenerative therapy and immune modulation [9], and more are ongoing. Table 1 summarizes currently available clinical trials registered with clinicaltrials.gov that are investigating CB and CB-derived products for regenerative purposes. Most are studying the use of donor, allogeneic CB administered as unmanipulated whole CB (total nucleated cells or TNC), mononuclear cells (MNC) or CB-derived mesenchymal stromal cells (MSC). The most common method of delivery is intravenous infusion, presumably based on both ease of administration and the assumption that the CB cells distribute throughout the body via the blood and affect the intended disease process through the release of chemical signals.
| Table 1: Ongoing clinical trials registered with clinicaltrials.gov utilizing CB for regenerative medicine purposes. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical category | Number of studies | Conditions | Cell source | Cell type | Route of administration | Phase of studies | ClinicalTrials.gov IDs | |||||||||
| Auto | Allo | TNC/ MNCs | MSCs | Other | IV | Direct injection | Other | NR | 1 | 2 | 3 | NR | ||||
| Neurology | 18 | Alzheimer’s disease Autism spectrum disorder Carbon monoxide poisoning Cerebral palsy Hypoxic ischemic encephalopathy Inherited metabolic diseases Intraventricular hemorrhage Stroke | 8 | 11 | 14 | 3 | 1 | 13 | 5 | 0 | 0 | 6 | 10 | 1 | 1 | NCT03004976 NCT02599207 NCT02433509 NCT02256618 NCT02455830 NCT01072370 NCT02612155 NCT01988584 NCT01673932 NCT02551003 NCT03087110 NCT02866331 NCT02952716 NCT02054208 NCT02254863 NCT02890953 NCT01929434 NCT02847182 |
| Hematology/ oncology | 6 | Leukemia/lymphoma Multiple myeloma | 0 | 6 | 0 | 0 | 6 | 6 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | NCT01362452 NCT01630564 NCT02280525 NCT02328885 NCT02781467 NCT02955550 |
| Endocrinology | 4 | Diabetes, type 1 Infertility | 0 | 4 | 0 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 3 | 0 | 1 | NCT02932826 NCT03011021 NCT02313415 NCT03033277 |
| Pulmonary | 4 | Acute lung injury Bronchopulmonary dysplasia Pneumoconiosis Radiation-induced pulmonary fibrosis | 0 | 4 | 0 | 4 | 0 | 1 | 1 | 2 | 0 | 2 | 2 | 0 | 0 | NCT02444455 NCT02381366 NCT02668068 NCT02277145 |
| Dermatology | 3 | Epidermolysis bullosa Psoriasis vulgaris Skin/sweat gland injury | 0 | 3 | 1 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | NCT01033552 NCT02491658 NCT02304562 |
| Infectious disease | 3 | HIV Infections of prematurity | 1 | 2 | 2 | 1 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | NCT02923076 NCT02140944 NCT02999373 |
| Cardiovascular | 2 | Chronic ischemic cardiomyopathy Hypoplastic left heart syndrome | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | NCT02635464 NCT01883076 |
| Gastroenterology | 2 | Crohn’s disease Ischemic biliary lesions | 0 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | NCT02000362 NCT02223897 |
| Opthalmology | 2 | Keratopathy | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | NCT03084861 NCT03064984 |
| Orthopedics | 2 | Anterior cruciate ligament injury Articular cartilage defects | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | NCT02755376 NCT01733186 |
| Other | 4 | Aging Aplastic Anemia Prematurity Vascular foot ulcers | 1 | 3 | 2 | 1 | 1 | 3 | 0 | 1 | 0 | 3 | 0 | 1 | 0 | NCT02418013 NCT03055078 NCT03053076 NCT02389010 |
| Total | 50 | 11 | 40 | 20 | 17 | 13 | 32 | 10 | 7 | 1 | 19 | 22 | 3 | 6 | ||
| If studies included combined phases, the latest phase of the study is indicated. Auto: Autologous; allo: Allogeneic; IV: Intravenous; MNCs: Mononuclear cells; MSCs: mesenchymal stromal cells; NR: Not reported; TNC: Total nucleated cells. | ||||||||||||||||
Clinical trials of CB as regenerative therapies are being conducted throughout the world but are concentrated heavily in Asia and the USA (Figure 1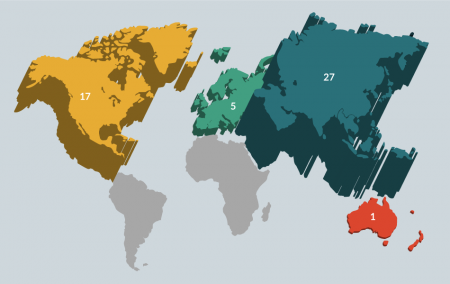
Potential neurologic mechanisms & applications of umbilical cord blood
Many neurologic diseases and injuries have long been considered irreversible and untreatable, with therapies focused on alleviating symptoms as opposed to treating the underlying cause. Traditionally, the brain was thought of as a static organ with an extremely limited ability to regenerate, repair or adapt to injury. Advances in neuroscience have challenged the conventional wisdom, however, discovering that the brain does indeed possess a limited capacity for self-renewal. This fundamental conceptual change has launched a new wave of research to determine how to harness and manipulate that ability to address previously untreatable neurologic conditions. Given the complex, intricate circuitry and microenvironments of the nervous system, biologic cell-based therapies have garnered substantial attention as potential treatments to repair damage, regain function and improve quality of life in patients with neurological disorders.
There are multiple mechanisms by which CB cells may affect cell and tissue repair in neurologic conditions, and different ones may be operative in different conditions or at different times during the course of injury and repair. Acutely, CB cells may deliver trophic factors that provide anti-inflammatory and neuroprotective effects thereby enhancing the survival of host cells [10-13]. They may also increase the plasticity of the injured and recovering brain by enhancing synaptogenesis, angiogenesis and endogenous repair mechanisms and/or by inducing migration and proliferation of existing neural stem cells [6,14,15]. To a lesser degree, CB cells may also migrate, integrate, proliferate, and differentiate into ‘replacement’ neuronal and glial cells and play a role in remyelination [16]. Finally, CB-derived cells could also potentially serve as a vehicle to deliver neuroprotective and restorative factors or signal endogenous cells to act in a targeted way toward damaged brain tissue. These mechanisms have been observed in numerous preclinical models of ischemic, genetic and neurodegenerative conditions of the central nervous system.
Currently, clinical trials of CB therapies are being conducted in patients with acquired brain injuries such as stroke (n = 3), cerebral palsy (n = 6), hypoxic ischemic encephalopathy (n = 4) and intraventricular hemorrhage (n = 1), genetic diseases that affect the brain (n = 1), neurodegenerative conditions (n = 1), and autism spectrum disorder (n = 1). The most common route of administration is intravenous, though intrathecal and direct injections are also practiced. Many published reports have suggested potential benefits [9], though few prospective, controlled studies have been completed.
The indication for which the most prospective data of CB therapy is available is cerebral palsy. In a randomized, double-blind, placebo-controlled trial of 63 children aged 1–6 years old with cerebral palsy conducted by our group at Duke University, there was no difference in motor improvement between the study groups as a whole. However, patients receiving a precryopreservation total nucleated cell dose of ≥2.5 x 107 cells/kg demonstrated greater improvement of motor function and normalized whole brain connectivity than subjects receiving smaller cell doses. This finding is consistent with two other randomized trials of CB therapy in children with CP, both conducted in Korea using allogeneic CB [17,18]. One of those studies demonstrated greater improvement on motor and cognitive scales in patients who received a precryopreservation cell dose of ≥3 x 107/kg + erythropoietin versus those who received erythropoietin alone or placebo. They also noted a dose correlation, with higher doses associated with greater improvement [17]. The second study showed similar results in 36 children randomized to treatment with CB or placebo [18]. Additional studies of CB for the treatment of cerebral palsy are being conducted in Australia, China, Korea and the USA.
Autologous CB infusions are being studied in newborn babies with hypoxic ischemic encephalopathy at birth. In a Phase 1 trial conducted at Duke, fresh, non-cryopreserved autologous CB infused in 1, 2 or 4 doses of 1-5 x 107 nucleated cells/kg within the first 72 hours of life in babies with moderate-to-severe encephalopathy qualifying for systemic hypothermia [19]. These babies were compared to a concomitant group of babies treated at Duke who were cooled but did not receive CB cells. Infusions were found to be safe in these critically ill babies, and babies receiving cells had increased survival rates to discharge (100 vs 85%; p = 0.20) and improved function at one year of age (74 vs 41% with development in the normal range; p = 0.05). A Phase 2 multicenter, randomized controlled trial is currently underway in the USA, and additional studies of CB infusion in babies with hypoxic ischemic encephalopathy are ongoing in China and Japan.
Other prospective randomized studies of CB infusion are being performed for adults with acute ischemic stroke and children with autism spectrum disorder. Infusion in these populations was shown to be safe in early reports [20,21], and the current investigations will shed light on the potential for therapeutic benefit.
Translational insight
Regenerative medicine represents a new frontier with the potential to transform the way diseases are understood, inspire innumerable lines of scientific investigation, and revolutionize the treatment of a variety of conditions for which current options are limited, inadequate or non-existent. CB is an attractive source of cells for such therapies due to its ease of collection, ability to be cryopreserved indefinitely, safe track record of use in the clinic and noncontroversial nature. In order to capitalize on these features and to make regenerative therapies available to the population at large, the development of allogeneic, off-the-shelf products that can be reliably manufactured, accessible and easily administered without the need for significant immunosuppression will be essential. Expanded indications for CB use in this manner would likely also necessitate changes in the current structure of public and private CB banking. However, data from additional human studies is needed to fully define the safety and efficacy of regenerative CB therapies and to determine the clinical applications in which its use is justified.
Financial & competing interests disclosure
The author has no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Regenerative Medicine: http://www.aabb.org/resources/bct/therapyfacts/Pages/regenerative.aspx. (Accessed December 11, 2013).
2. Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013; 122(4): 491-8.
CrossRef
3. Hess DA, Craft TP, Wirthlin L et al. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells 2008; 26(3): 611–20.
CrossRef
4. Huang CJ, Butler AE, Moran A et al. A low frequency of pancreatic islet insulin-expressing cells derived from cord blood stem cell allografts in humans. Diabetologia 2011; 54(5): 1066–74.
CrossRef
5. Gussoni E, Bennett RR, Muskiewicz KR et al. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J. Clin. Invest. 2002; 110(6): 807–14.
CrossRef
6. Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist 2003; 9(1): 64–75.
CrossRef
7. Kurtzberg J, Kosaras B, Stephens C, Snyder EY. Umbilical cord blood cells engraft and differentiate in neural tissues after human transplantation. Biol. Blood Marrow Transplant. 2003; 9(2): 128–9.
CrossRef
8. Hoogerbrugge P, Suzuki K, Poorthuis B, Kobayashi T, Wagemaker G, Bekkum Dv. Donor-derived cells in the central nervous system of twitcher mice after bone marrow transplantation. Science 1988; 239(4843): 1035–8.
CrossRef
9. Rizk M, Aziz J, Shorr R, Allan DS. Cell-Based Therapy Using Umbilical Cord Blood for Novel Indications in Regenerative Therapy and Immune Modulation: An Updated Systematic Scoping Review of the Literature. Biol. Blood Marrow Transplant. 2017; pii: S1083-8791(17)30498-6.
DOI
10. Llado J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol. Cell Neurosci. 2004;27(3):322-331.
CrossRef
11. Vendrame M, Gemma C, de Mesquita D et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14(5):595-604.
CrossRef
12. Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004; 35(10): 2385–9.
CrossRef
13. Arien-Zakay H, Lecht S, Bercu MM et al. Neuroprotection by cord blood neural progenitors involves antioxidants, neurotrophic and angiogenic factors. Exp. Neurol. 2009; 216(1): 83–94.
CrossRef
14. Chen J, Zhang ZG, Li Y et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 2003; 92(6): 692–9.
CrossRef
15. Taguchi A, Soma T, Tanaka H et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 2004; 114(3): 330–8.
CrossRef
16. Shen LH, Li Y, Chen J et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience 2006; 137(2): 393–9.
CrossRef
17. Min K, Song J, Kang JY et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells 2013; 31(3): 581–91.
CrossRef
18. Kang M, Min K, Jang J et al. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. Stem Cells Dev. 2015; 24(19): 2259–68.
CrossRef
19. Cotten CM, Murtha AP, Goldberg RN et al. Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. The J. Pediatr. 2013; 164(5): 973–9.e1.
CrossRef
20. Dawson G, Sun JM, Davlantis KS et al. Autologous Cord Blood Infusions Are Safe and Feasible in Young Children with Autism Spectrum Disorder: Results of a Single-Center Phase I Open-Label Trial. Stem Cells Transl. Med. 2017; 6(5): 1332–9.
CrossRef
21. Kurtzberg J, Troy JD, Bennett E et al. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke (CoBIS): Clinical Outcomes From a Phase 1 Study. Biol. Blood Marrow Transpl. 23(3): S173–4.
Webiste
Affiliations
Jessica M Sun
Robertson Clinical and Translational Cell Therapy Program, Duke University Medical Center, NC, USA.
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.