Challenges and advances in scale-up of label-free downstream processing for allogeneic cell therapies
Cell Gene Therapy Insights 2017; 3(6), 447-467.
10.18609/cgti.2017.041
Recent advances in stem cell research and regenerative medicine are leading towards the realistic commercial prospect of more complex cell-based therapeutic products, offering the potential to revolutionize aspects of healthcare system. To date however, there are no truly ‘large-scale’ cell therapy products available. To achieve successful commercial production, many factors come into play. To name a few; economics, robustness, reproducibility and, what this review is concerned about: scalability. With cell therapies, a change in the processing environment may lead to a product change, which ultimately may be the difference between a successful batch (meeting product specifications) or a failed one [1]. To minimize process changes throughout the scales, processing steps must be carefully selected from an early stage. A particular challenge faced is that current ‘gold standard’ techniques for cell separation are not generally compatible with large scale processes. Dead-end batch centrifugation is a clear example of a process step that is heavily manual, difficult to automate while maintaining sterility, and limited in scalability [1]. The scope of this article is to explore and evaluate current and potential future techniques for cell separation at large scale only.
Scalable downstream processing for allogeneic cell therapies
Scale-up of cell manufacture can be achieved horizontally, through scale-out (serial production with multiple devices and/or production sites) and vertically, through scale-up (increasing the volumetric or cell number throughput of a single device). On the one hand, scale-out manufacture is likely to suffice for patient-specific therapies, such as many of the many immunotherapies, or even rare diseases therapies, for which small production batches are needed. On the other hand, cell therapeutics that require scalable manufacturing solutions are off-the-shelf (allogeneic) therapies. These can encompass therapies such as those that use pluripotent stem cells (embryonic, mesenchymal or induced pluripotent stem cells), immortalized progenitors, or differentiated cell populations. For these types of therapies, large scale can range anywhere from 50 L to a few hundred liters. In this review, when defining a technology as ‘capable of handling large scale’, it refers to a minimum of 50 L with potential of handling a few hundred liters. Moreover, the term ‘scalable’ is used to refer to technologies suitable for scale-up to address the allogeneic therapies’ needs. Scale-out sits outside of the scope of this review.
To properly identify the potential of a downstream processing technique, we propose a series of parameters to be considered when assessing the suitability for large-scale cell manufacture; namely whether or not its label-free, process mode (batch vs continuous), containment (closed vs open) and resolution (low vs high).
Current challenges with traditional downstream techniques for cell-based therapeutics
Cell-based products create new challenges for large scale production and processing, necessitating a re-think of current manufacturing strategies. With these therapies, the end products (i.e., the cells themselves) are defined by their respective manufacturing process [1]. Therefore, exposure to mechanical and physiochemical stresses during processing can ultimately lead to a change in identity of the end product. To add to the challenge of maintenance of identity throughout processing and scales, there is also the number of cells required for a therapeutic effect.
The number of cells that compose a clinically relevant dose will differ for varying therapies. For instance, a clinically relevant dose of human pluripotent stem cell (hPSC) derived cardiomyocytes for myocardial infarction is estimated at 1×109 cardiomyocytes [2], which is similar to that estimated for bone marrow-derived mesenchymal stromal cells (MSCs) for graft-versus-host disease (GvHD) [3]. On the other end of the scale, a single unit for red blood cell transfusion requires approximately 1012 cells. This means that a single dose requires the production of 200 L at a density of 5×107 cells mL-1 or, if one were to control the final volume to something less risky and more manageable, such as 5 L, this would require a final cell density of 2×1011 cells mL-1. Both scenarios present challenges for traditional downstream processing systems. Processing 200 L of cells with current technologies can take hours, compromising cell health by holding the cells prior- and post-processing which can lead to increased cell death [4]. Processing a smaller volume with very high cell density (i.e., highly viscous cell suspension) will inevitably be harder to handle.
Another key consideration in the manufacturing of any cell-based product is its purity and the degree of contaminant removal needed. In the case of stem cell-derived therapeutics, this encompasses freedom from unknown or adventitious pathogens, maintenance of cell quality attributes, removal of additives and secreted products from the expansion phase, through to the homogeneity of cell populations. Ensuring cellular homogeneity is key throughout a manufacturing process to ensure robustness and reproducibility of protocols as well as purity of the intended end product. Regardless of whether the product is intended to be used in vitro (discovery or screening) or in vivo (for transplantation to treat a specific indication), homogeneity will remain a key target in the product specification to ensure efficacy and reproducibility. Moreover, a critical concern for cell therapies will be the absence of undifferentiated cell populations in the final cellular product intended for transplantation. These cells have the potential to form teratomas: benign tumors capable of interfering with tissue physiology, or worse, teratocarcinomas with metastatic potential. Consequently, there exists a pressing manufacturing need for effective separation technologies that can meet the stringent target criteria.
Downstream processing methodology design
Development of downstream processing technologies suitable for cell manufacture must balance the requirements of the intended product (e.g., composition, purity, cell quality attributes, etc.), as well as the demands of the process as a whole (e.g., sample preparation and handling, running costs, ease of operation, skilled personnel requirements, Good Manufacturing Practice compatibility, etc.).
With this in mind, the mode of operation and methodology employed can become crucial in designing a suitable manufacturing strategy. Due to the varied nature of cell therapy products, different desirable traits will be key for some type of products and may not necessarily be as key for others. This section will discuss some considerations that should be taken into account when choosing downstream processing steps for any allogeneic cell therapy workflow (Figure 1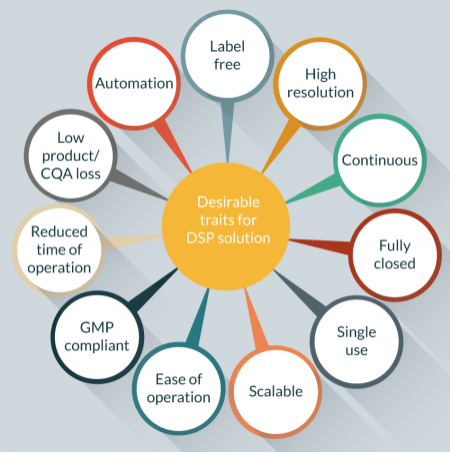
Label versus label free downstream processing
Labeling methods are those that rely on a tag for separation. Separation may be positive (where the cell of interest is the main target either by carrying the label, or by being targeted by a component carrying the label), or negative (where the cell of interest is not the main target and the label aims to retain a different component).
The two main cell separation methods that rely on labeling and are widely used for research and clinical practices are flow cytometric fluorescent-activated cell sorting (FACS) and magnetic activated cell sorting (MACS) technologies. Fluorescent- and magnetic-activated cell sorting methods are based on the recognition and labeling of cell-associated epitopes located inside or on the surface of cells. Detection normally relies on targeted epitopes using high-affinity probes including antibodies, peptides or nucleic acids directly or secondarily labeled with photo-excitable and light emitting molecules or nano- or micro-sized particles. These techniques have proven very useful for a variety of applications and have the potential to yield highly pure cell populations (>95%). However, cell labeling adds process time and costs, and the success of high affinity labeling is dependent on the strength and exclusivity of expression of the targeted epitope used to discriminate cell populations. In addition, in the case of positive selection, labels may need removal depending on application, and incomplete label removal may have implications for therapeutic use. But even in the case of negative selection, label-requiring methods may not be optimal for many clinical applications, as antibodies and labels (dyes, magnetic particles) have been introduced into the sample at the start of the procedure, and may still be present in the enriched and depleted cell fractions.
Although label-requiring downstream methods are clearly a key part of future cell therapy manufacture, the demand for cell selection techniques more compatible with large-scale clinical application has resulted in the development of a variety of label-free cell separation technologies, explored in this review. For the purpose of this review, only label-free technologies will be assessed. The authors believe that label-free techniques have scope for scale-up whilst labeling technologies may be more suitable for small batches and patient-specific therapies.
Whilst label-free techniques do not present the above-described drawbacks, utilizing cell biochemical or biomechanical properties (e.g., size, polarity, charge, elasticity or deformability), may not be specific enough and can result in compromised cell purity, selectivity and yield.
Label-free downstream techniques currently in research use or under development are mostly either derived from the field of biomicrofluidics (and have thus far primarily been designed for small-scale use like cancer diagnostics and drug monitoring rather than large-scale cell separation for therapeutics) or are adapted from unit operations more traditionally used in protein therapeutics (and therefore may not be sufficiently tailored to cellular targets to offer selectivity). To offer significant progress in this field, new techniques should aspire to be easily scalable whilst offering improved selectivity.
Low versus high resolution
Resolution refers to the capacity of the downstream processing platform to distinguish the cell of interest from the remaining components. Different techniques will offer varying degrees of resolution. Ultimately, the resolution requirements for the downstream platform will be largely dictated by the nature of the starting population. Low resolution might suffice if the starting population consists of distinctly different cells (as would be the case e.g., for red blood cell or plasma products), while populations made up of very similar cells or containing rare cells would demand high resolution (e.g., selection or removal of hematopoietic stem cells and cancer cells).
Containment
Containment (open or closed) refers to the extent to which cells are exposed to ambient environments. A ‘closed’ environment is limited by the boundaries of the vessel or device the cells are contained in. While these boundaries may be gas permeable, they otherwise constitute barriers to pathogens or other non-gas permeable contaminants in the atmosphere. Open systems will require the use of regulated environments (i.e., laminar flow hoods, ‘clean rooms’) that control and limit airborne contaminants below that normally detected in ambient atmosphere. Creating, operating and maintaining such environments is very costly, and increases significantly with scale, but are essential to comply with regulatory standards. Environmental specifications for operators of closed systems are substantially lower, thereby reducing costs and risk of contamination to the final product.
Batch versus continuous separation
Batch operation is when there is a defined start and end to a process, whereas continuous is when production is on-going and the product is constantly being produced. A key advantage of batch processing is the ability to accurately distinguish the start and end of the production of a batch, which is desirable from a regulatory perspective. However, often batch processes require one or more ‘hold’ steps to match processing times to equipment, which is non-ideal for cell therapy products. Every batch has to be tested and qualified to ensure inter-batch reproducibility and for safe product release. Quality control (QC) tests can often be expensive (generally due to the expensive nature of the product being tested) and lengthy, and generally the product cannot be used until QC results are satisfactory for safe release.
Continuous production allows for larger amounts of product being manufactured, and thus is preferable for allogeneic therapies. Whereas it becomes less clear with respect to QC testing, if the production can be validated to meet product specifications for a given period of time, then QC burden diminishes. However, there’s an inflection point at which the risk associated with something going wrong during processing outweighs the benefits of continuous production.
Scalability
Of particular interest in cell therapy development are separation methods that may lend themselves to large-scale production, particularly through scale-up. Easily scalable separation techniques will become more and more important as regenerative medicine develops, and particularly as allogeneic therapies become more common. Moreover, the development of bench-scale technologies that better mimic the full scale will be paramount to the rapid development of robust downstream processes and allow cost-effective process optimisation [5]. Many of the currently applied downstream processing techniques, whilst highly effective in terms of cell isolation, are limited by the need to label target cells to effect isolation and by a limited maximal scale of operation.
Downstream processing step positioning
A further driver for methodology selection is where in the process workflow to implement the required downstream manufacturing process. Unlike more traditional biotherapuetic processes, it may not be necessary, or even optimal, to purify at the end of the process, but rather to consider purification steps at key points. For instance, if the challenge is removal of pluripotent cells from the product, a purification step immediately after lineage commitment during a differentiation may be simpler and could certainly be carried out at smaller scale than a step at the end of expansion phase post-commitment.
Currently developed downstream processing techniques
In this section, a selection of reported downstream processing techniques potentially suitable for allogeneic large-scale clinical production are considered. Only label-free technology that can be scaled-up, or has the potential to do so, will be reviewed. For each separation method the underlying technical principle is described, followed by discussion of the above listed selection criteria. Techniques that do not meet all the selection criteria (such as spinning membrane filtration and others) will not be reviewed.
Field flow fractionation separations
This category of separation techniques look to use waves or fields to create landscapes that drive cells to migrate to certain points, based upon key properties of the cells. In general, these methods combine a laminar flow with a perpendicular, axial radiation force to create field-driven separation.
In terms of scale-up, all field flow fractionation (FFF) systems present similar challenges: the separation is 1D and therefore generally constrained in throughput. The development of 2D and 3D fields overcome this challenge and are the predominant features in the most recent techniques developed (discussed below).
Acoustophoresis
Acoustophoretic sorting separates cells based on cell size, density and compressibility. It is a label-free method that employs the interaction between the acoustic radiation force exerted by an ultrasonic standing wave and the fluid drag force on the cell. The acoustic radiation force will result in the particle moving toward either the pressure node or antinode of the ultrasonic waves, depending on the size of the cell and its deformability (determined by both the cell and fluid density and compressibility). Acoustophoresis has been described as a gentle separation method with no apparent acute adverse effects for the cells due to exposure to ultrasound, including possible non-thermal effects due to cavitation ([6] and references therein). The reported separation resolution is high, and can be optimized for required cell type by adjusting operating settings (Figure 2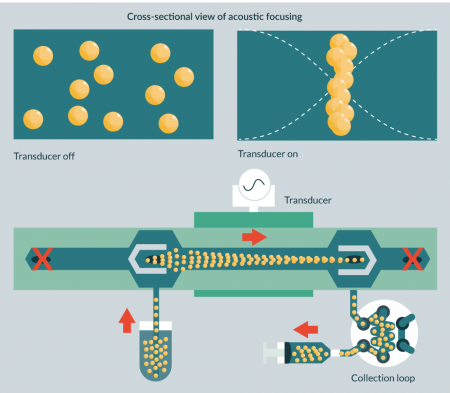
Acoustophoretic sorting devices had traditionally been developed as small, microfluidic platforms, not amenable to high throughput unless operated in parallel. Moreover, increasing sample flow rates in an attempt to increase throughput per device reportedly reduced recovery efficiency [7]. However, recent progress has seen the appearance of a couple of commercial acoustophoretic separation technologies – one from Applikon® Biotechnology and one from FloDesign. These technologies are used as perfusion devices in stirred tank reactors as well as for downstream processing (cell washing and concentration). BioSep (Applikon® Biotechnology, Netherlands) uses SonoSep’s acoustic wave separation technologies, based on a planar standing wave. Whereas most reported findings show promising results with CHO and insect cells [8], available literature for cell therapy applications is limited. For the SonoSep device, the cells are held by in the chamber during perfusion, whilst a harvest flow is drawn through to the waste. The range of application for perfusion purposes spans from 1 to 1000 L, yet its scalability with respect to downstream processing applications may not be as comprehensive. The size of the chamber becomes the bottleneck as it can only hold so many cells before they start aggregating due to cell–cell contact.
The newer generation of this technology is the FloDesign Sonics device (FloDesign Sonics, USA), which uses a multidimensional standing wave instead of a planar one. The 3D field acts as a membrane; similar methodology to crossflow filtration but without the use of a physical membrane. This particular device is used for perfusion and cell concentration. In the perfusion mode of operation, the acousto-fluidic interaction mimics that of tangential flow filtration, where the acoustic radiation force prevents the cells from entering the acoustic field and thus return to the bioreactor or other culture vessel. The range of applications for perfusion purposes spans from 0.25 to 100 L. For concentration, the cells are trapped in the standing wave, allowing up to 40×109 cells at flow rates of 2 L every hour to be processed. The particular set-up explained for the perfusion mode allows for much faster flow rates of media or buffer exchange and does not present the same problem with the chamber size being the limiting factor for the downstream processing, as the process is taking place in-situ (whether it is a culture vessel or a hold tank). The concentration mode is carried in the chamber to achieve high cell densities in a small volume.
Dielectrophoresis
Dielectrophoresis (DEP) selects cells based on a variety of parameters, including size, shape, cytosol conductivity, polarizability and membrane properties like permeability, capacitance and conductivity. DEP-based separation is a label-free method whereby an electric force is exerted on dielectric particles, including cells suspended in a non-uniform electric field. The strength of this exerted force is dictated by properties of not only the cells but also the media, as well as the frequency of the electric field, and dictates whether a cell is attracted to or repelled by the charged electrode. Adjustment of the DEP force field can be obtained through an abundance of tunable factors, allowing manipulation of the cells according to various cell intrinsic properties. The technique has been used to separate a variety of mammalian cell types, including cancer cells [9], infected red blood cells [10] and undifferentiated pluripotent stem cells from their differentiated progeny [11,12]. Though DEP has been successfully integrated into a commercially available cell selection technology (i.e., ApoStream®, from ApoCell), this system is currently being used to select rare cells (e.g., circulating tumor cells) from a population, so distinct from separation in the context of a cell production processes. Indeed, a number of bottlenecks have been raised that likely prevent scalable DEP-based separation platforms for cell manufacture. Heating near the electrodes due to media conductivity may result in bubbles and heat-induced cell death, or the generation of free radicals. Additionally, the strong dependency of DEP on cell size means that inter cell-type size variation may override cell type specific differences, reducing the relevance of the technique. The latter hurdle, however, might be overcome with isoelectric DEP, where sorting is mainly dependent on the dielectric properties rather than size by using a media conductivity gradient[13]. Potentially, DEP could be part of a closed, continuous, cell separation platform that could achieve large-scale selection through scale-out rather than scale-up, by placing multiple devices in parallel.
Optical landscapes

Optical sorting of cells is a technique based on cell size and refractive index using an optical force. Depending on the type of optical sorting method, cell labeling is essential, or optional, merely enhancing selectivity. Dholakia et al. call the former active, and the latter passive optical sorting [14]. We will not delve into active sorting, as it requires a label and therefore is outside of the scope of this review. Passive optical sorting can be achieved with optical tweezers [15], optical chromatography [16] or optical energy landscapes [17]. Optical tweezers use radiation pressure from a focused laser beam to trap and move cells, while optical chromatography employs the balance of fluidic and optical forces on a particle in a channel with a flow of fluid in the opposite direction of a laser beam. Optical energy landscapes use the interference of two or more laser beams to generate an energy landscape, and utilize variation in shape, size and refractive index to induce separation movement in different populations within the landscape. Though optical sorting has been used successfully to separate distinct mammalian cell populations [17,18], this can only be achieved when the differences in size and refractive index of the various populations is large enough [19]. Moreover, separation efficiency might be compromised due to the fact that a cell could have different refractive indexes depending on the position or state of its various components. To date, optical separation in literature has been achieved only with small-scale devices. However, the development of larger scale and 3D optical landscapes is a focus of current research, and this approach may render the methodology suitable for adaption to larger scale downstream processing techniques, particularly if power consumption can be addressed.
Centrifugal techniques
Centrifugal separation of cells is far from a new concept. To date, the cell therapy industry workhorse is the swing-bucket rotor centrifugation (batch dead-end centrifugation), which has proven to be difficult to automate and -as it stands- is not a scalable solution [1]. For this reason, it falls outside of the scope of this review. However, the underlying principle of centrifugation has been used in the design of novel cell processing methodologies and integrated automated platforms that combine centrifugation and filtration. Centrifugal separations employ size and density variations to achieve separation, driven by centrifugal acceleration.
Centrifugal counterflow elutriation

Centrifugal counterflow elutriation (CCE) involves cells in a conical centrifugal chamber being subjected to centrifugal forces in an outward direction and a fluid force in an inward direction. It exerts gentle force on the cells, making it an attractive downstream processing option (Figure 3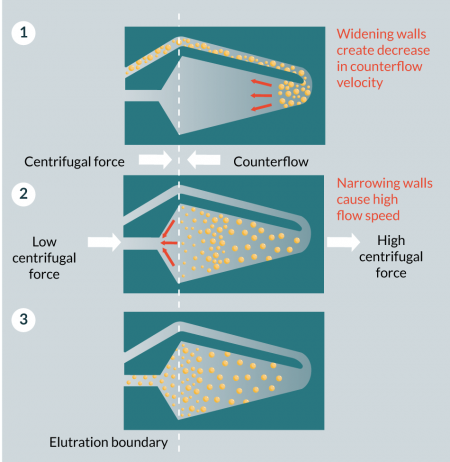
Since its introduction in the 1970s, CCE has been used routinely for research purposes, and later also for clinical procedures, including plasmapheresis and the collection of particular white blood cells and stem cells [20-25] from apheresis products. Commercially available CCE platforms include the JE-5.0 Elutriation System (Beckman Coulter, USA), Elutra© Cell Separation System (Terumo BCT, UK) and kSep® Systems (kSep400, kSep6000S and beta testing smaller sized unit for process development, from Sartorius Stedim, UK).
Within these platforms CCE is already part of an automated, closed, integrated cell separation process capable of washing and then separating large batches of heterogeneous cell populations. For example, the capacity of Elutra© processing chamber is 3 x 1010 white blood cells, while the kSep6000S is able to separate up to 1200 x 109 cells at 10–50 x 106 cells mL-1 in a single use, fully closed, automated GMP-compatible platform. Whether CCE, and these commercially available systems in particular lend themselves equally well for the separation of undifferentiated pluripotent stem cells from their in vitro differentiated progeny needs to be determined. With these technologies capital cost can be high, but one machine can potentially accommodate a large range of production scales, which may make a strong case for investment.
Centrifugation itself is relatively easily scaled, and as such should present a strong case for incorporation in large scale downstream processing for allogeneic cell therapies. There are, however, two challenges to this. Firstly, CCE is not strictly a continuous centrifugation operation. The necessity of maintaining the density gradient within the separating chamber means that larger scale requires larger chambers, not increased continuous throughput, and this rapidly becomes problematic from a rotational stability point of view. Furthermore, as the size of the device increases, physical shear forces on the cells during introduction increase, running the risk of damage to sensitive cell products.
Continuous centrifugation with flexible diaphragm
During centrifugation, the flexible diaphragm located inside the centrifuge bowl can be inflated with hydraulic fluid. As the diaphragm inflates, it presses against the bottom of the cell processing bag giving these techniques the capacity to express fluids and/or cells for removal or collection during centrifugation. Two commercially available automated centrifuge systems for blood component processing are the TACSI® PL and the COBE® 2991 Cell Processor (Terumo BCT, UK).
TASCI® PL (Terumo Automated Centrifuge and Separator Integration System for Platelets) is a closed, integrated manufacturing system that can process up to six buffy-coats at a time into platelet concentrate by first pooling and centrifugation of the buffy coats followed by pump-operated filtration for separation and leukoreduction of the final platelet product. Reports show that the system, which is used most commonly in blood centers, achieves efficient platelet recovery [26] suitable for clinical use [27].
The COBE® 2991 Cell Processor contains a centrifuge with a flexible diaphragm and separates components based on specific gravities. It is an integrated system whereby a hydraulic fluid presses against a flexible membrane to express fluid or cells into an attached collection bag. It is a versatile system that can be used for blood and cell sample washing, volume reduction of cell products and cell concentration. For specific cell selection purposes, for example human pancreatic islet isolation, however, it does not seem to improve on manual density gradient purification [28].
In terms of scalability and engineering challenges, these systems are similar to the CCE approach described previously. However, it should be easier to develop a continuous version of a centrifuge/filter system, potentially simplifying scalability.
Hydrodynamic-type techniques
Broadly, these types of separation methodologies rely on flow behaviors to separate cells according to various properties. Here we have loosely grouped them, although individual techniques rely on differing effects to drive separation.
Deterministic lateral displacement

Deterministic lateral displacement (DLD) is a label-free, passive technique that separates cells according to size and deformability (Figure 4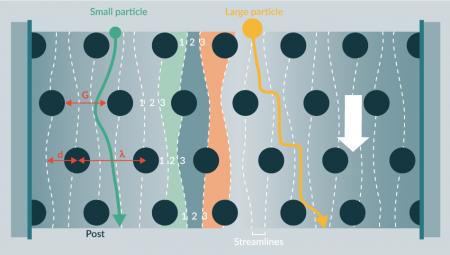
Potentially DLD could be scaled up, but there are manufacturing challenges in constructing large micropillar arrays and significant problems with blockages.
Although DLD’s simplicity is attractive, and DLD cell separation achieves good size resolution [30] and high enrichment [31], clogging of the device could be an issue for its implementation in an integrated, closed separation platform for cell manufacture. This is particularly likely to be the case in a sample containing large ranges of cell sizes, as DLD devices tend to be inaccessible to particles significantly larger than their chosen separation diameter. However, reducing the pillars to point-like obstacles (thus reducing fluidic resistance) may improve throughput [32]. Furthermore, DLD is still attractive as a final “polishing” step where contaminants may be low in number and similar in properties to target cells.
Hydrodynamic filtration

Hydrodynamic filtration (HDF) selects cells according to size and shape [33]. HDF is a method whereby cells in a continuous flow are separated based on their flow profile as dictated by their size, which subsequently affects their proximity to the channel wall. HDF devices contain a main flow channel with a number of sideway collection outlets. Smaller cells (which will flow closer to the channel sidewall than larger cells) will enter the outlets earlier, and are thus the first to leave the channel. Separation is based purely on cell flow profile and not channel geometry. This means channels can be a lot larger than the cells, reducing clogging and increasing throughput, which is theoretically advantageous for scalability. The main drawback is that laminar flow profiles must be maintained, which becomes more challenging with larger diameters. The method has been used successfully to separate various cell types, including leukocytes [33] and liver cells [34] and, using HDF devices with curvilinear geometry to add Dean drag force, blood cells from plasma and white blood cells from red blood cells [34]. HDF devices achieve high separation efficiencies of 95% [35] at high throughput rates ranging from 2 x 105 to 1 x 106 cells min-1 [34,35]. To date, devices are small scale and are being optimized for point-of-care applications rather than large-scale manufacturing [35].
Inertial migration

Inertial migration is a size-based cell separation method. A label-free, active technique that makes use of intrinsic hydrodynamic forces (Figure 5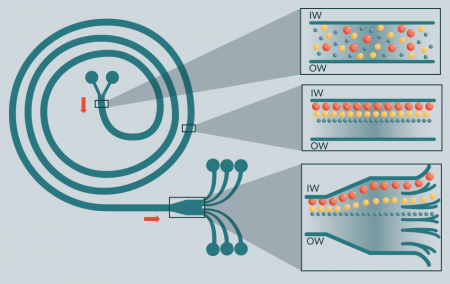
Two types of inertial migration devices have been designed, consisting of circular pipes and/or spiral channels (asymmetric curvilinear channel). In both designs, cells are focused thanks to the balance of two inertial lift forces (known as ‘tubular pinch effect’): shear gradient lift; and wall effect lift. In the curved-channel design, Dean drag force is added as a third force, allowing multi-size separation. As a result, cells of a particular size are focused in a unique equilibrium position, generating distinct particle streams, which are collected in separate outlets at the end of the channel. Separation efficiency of this technique is inherently linked to cell population size heterogeneity. It has been shown to reach 80% separation when used to distinguish two types of neuronal cells, neuroblasotoma and glioma cells [36]. Due to its relatively simple design, the technique can be implemented as a closed, integrated platform for continuous cell selection. Similarly to HDF, the channel width can be much larger than the cell sizes, allowing high volumetric flow rates (>106 cells min-1), while reducing the chance of clogging.
Jung et al. have developed various multi-orifice flow fractionation (MOFF) devices for continuous cell separation, which utilize inertial lift and momentum-change-induced inertial force generated in a series of contraction/expansion microchannels, and show they can be used to efficiently select rare cells from blood samples (e.g., circulating tumor cells). However, to date, these devices have not been reported for use in large-scale cell separation [40,41].
Filtration-based techniques
Filtration techniques are well established within the pharmaceutical industry and therefore there is a lot of expertise around this topic. The most common types of filtration are normal flow filtration (otherwise known as dead end), tangential flow filtration (or cross flow) and spinning membrane filtration. Spinning membrane filtration techniques are outside of the scope of this review, given that they are not amenable to large-scale manufacturing.
Filtration principles generally rely on differences in size for separation, which limits the ability to highly resolve cell populations, as heterogeneity of cells usually means that the size range of cells of a single type overlaps any difference in sizes between cells of different types. However, recent progress has seen elastic modulus and other parameters used as well as size to affect separation, and in some specific applications (such as blood fractionation), size alone may be an effective separation parameter.
Normal flow filtration

Normal flow filter devices contain small filters (sieves) with pores or holes to selectively capture cells based on size and deformability. Since these devices are not reliant on an external force or cell labeling, they can be used as a simple dead-end filter, trapping unwanted cells. As a consequence, the method works best if the percentage of unwanted cells is relatively low, so as not to clog the filter too quickly. In addition, clogging of the pores may result in flow irregularities. The platform has been used for research and diagnostics in the cell therapy area (e.g., trapping of rare circulating cancer cells) but not for (large-scale) cell production for the clinic. However, normal flow filtration (NFF) for cell removal is routinely used at very large scale in the manufacture of protein therapeutics from mammalian cells, so clearly there is scope for NFF to work as a scalable technique.
In general, NFF would only be suited for negative selection processes, and then only when seeking to remove larger contaminating cells from smaller target cells, but may present an interesting opportunity in some processes, particularly as a more crude early-stage separation technique.
Tangential flow filters
Tangential flow filters (TFF; also known as cross-flow filters or membranes) select cells based on size and deformability during laminar flow migration through porous membranes or other filtration arrays (Figure 6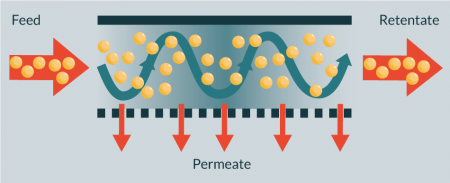
The technique has been used to isolate and enumerate cancer cells from blood (isolation efficiency >80% by [43]), for blood separation (e.g., >95% red blood cell removal by [44]), and to recover >97% plasma selectivity when separating plasma from whole blood [45]. However, the reported devices are microscale, and samples require rigourous dilution (>50 times [44]) prior to separation. Zhang et al. describe in their proof-of-principle paper successful separation of two cancer cells with different deformability using a TFF chip device, but with limited efficiency (the fraction of the more elastic cells increased from 50 to 73%). Willoughby et al. reported similar results when separating pluripotent and differentiated osteoblastic cells, and pluripotent cells and fibroblasts[46, 47]. A study comparing various silicon-based microfilters to separate white blood cells from red blood cells indicates that TFF is superior over NFF and weir and pillar type filters [48]. TFF systems are commonly operated at large scale in the mammalian and bacterial cell culture industries and several companies produce large-scale TFF membranes suitable for handling mammalian cell material.
At a larger scale, the use of TFF as a continuous, integrated alternative techniques for the washing and concentration of human mesenchymal stem cells (hMSCs) has been reported [49]. Using this technique, Cunha and colleagues [49] showed high protein clearance (98%), and high recovery of viable hMSCs (70%) with no impact on cell viability (95%) or other CQAs (morphology, immunophenotype, proliferation, adhesion capacity and multipotent differentiation potential). Furthermore, these studies show shorter processing times when using continuous filtration techniques as opposed to discontinuous operations. As is clearly portrayed in Cunha’s report, care must be taken during scale-up to ensure that shear levels within the flow path of the membrane systems remain low enough to not adversely affect cells.
Simple versions of TFF separations can be seen in weir and pillar type devices, which force cells past obstacles to separate on the basis of size and deformability, as above. Both weir-and pillar-type filters have been reported as part of a continuous flow device [50]. So far studies on fractionating whole blood report low flow rates and limited efficiency and a study by Ji et al., comparing TFF, NFF, weir-type filters and pillar-type filters for the separation of white blood cells from red blood cells showed TFF as being more efficient that the latter three [48]. Weir-type and pillar type filters may thus be more suited for small scale applications, for example obtaining a relatively low number of particular cells for downstream analysis rather than large-scale cell production processes.
Expanded bed chromatography

Expanded bed chromatography (EBC) is another well-established downstream technique. Its use for cell therapies has been limited yet promising. Cunha et al. have reported successful use of negative mode EBC, with a multimodal prototype matrix based on core-shell bead technology, for hMSC downstream processing [50]. The study reports an expanded bed with a stable and characterized matrix using standard equipment adapted from what was previously used for conventional packed bed chromatography processes. This technique shows improved washing and concentration of hMSCs compared to the previous TFF report [42], achieving higher recovery of viable hMSCs (89% compared to 70%) with no impact on cell viability (95%) or other CQAs as mentioned in the last section.
Admittedly, with this technique, there are trade-offs to be made between cell recovery and protein clearance, but it can be efficiently integrated with already existing downstream processing to improve washing efficiency up to 10-fold whilst maintaining high hMSC recovery of 70% [50].
| Table 1: A summary of considered techniques. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overarching category → | Field flow fractionation | Centrifugal techniques | Hydrodynamic-type techniques | Filtration-based techniques | Expanded bed chromatography | |||||||
| ↓ Trait ↓ | Principle → | Acoustophoresis | Dielectrophoresis | Optical landscapes | Centrifugal counterflow elutriation | Continuous centrifugation w/ flexible diaphragm | Deterministic lateral displacement | Hydrodynamic filtration | Inertial migration | Normal flow filtration | Tangential flow filters | |
| Containment | Open vs Closed | Closed | Closed | Closed | Closed | Closed | Closed | Closed | Closed | Closed | Closed | Closed |
| Label | Label vs label-free | Label-free | Both | Both | Label-free | Label-free | Label-free | Label-free | Label-free | Label-free | Label-free | Both |
| Process mode | Batch vs Continuous | Continuous | Continuous | Batch | Both | Batch | Continuous | Continuous | Continuous | Batch | Continuous | Continuous |
| Resolution | Low vs High | High | High | High | Low | Low | High | Low | High | Low | High | High |
| Availability | Commercially available vs In development | Commercially available* | Commercially available | In development | Commercially available | Commercially available | In development | In development | In development | Commercially available | Commercially available | Commercially available |
| Ease of scale-up | +++ Easy | ++ | + | + | +++ | +++ | + | + | + | ++ | +++ | +++ (negative mode & membrane adsorbers) ++ (conventional packed bed) |
| ++ Medium | ||||||||||||
| + Hard | ||||||||||||
| Note that high scoring in every category, does not necessarily mean the best available technology, as some of the scoring has been done based on potential for development a technique may hold and not only current state. | ||||||||||||
Translational insight
To bring cell-based therapies from bench to clinic, cost effective, scalable downstream processing techniques with high-resolution are required for integration into GMP-compatible bioprocessing platforms. Whilst this is the case for cellular therapies in general, it must be considered a particular priority for allogeneic therapies likely to be manufactured on larger scale for multiple recipients. Though various new cell selection methodologies described in this review are promising candidates that can achieve high purity and selectivity, at the current level of development trade-off on various traits must usually be accepted. Many technologies are currently more suited as point-of-care devices until further scalability has been addressed. Indeed, to date few can be seen to clearly perform better in terms of combined selectivity and throughput than conventional labeling methods. However, these established labeling methods do propose certain drawbacks that make them non-ideal for clinical applications. Moreover, due to their batch-type operation they do not lend themselves to be easily incorporated in integrated cell manufacturing system and may be limited in terms of scale of operation. As far as we are aware, the continuous centrifuge-based cell processing platforms described in this review as well as the later generation of acoustopherisis devices, are to date the only label-free commercial cell handling devices that have demonstrated processing of clinical grade cellular products in closed systems.
Undoubtedly, the reviewed methods will require further bioengineering development and need to evolve from their current small-scale chip design to devices capable of high throughput to overcome the rate-limiting step – efficient cell selection – of bringing regenerative medicine, and especially stem cell-based therapeutics, to the patient. Given the number of methodologies under investigation, and the ‘usual’ requirement in downstream bioprocessing to operate with multiple distinct separation steps, development of hybrid devices; combining advantageous aspects of various methodologies, is likely to prove a useful approach. Lastly, due to the infancy of the cell therapy industry, disruptive technologies have a lot of potential as no technology has yet established itself as the gold-standard for the new products handled by this rapidly growing industry.
financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Disclaimer
This article is adapted from Chapter 5 of the book ‘Bioprocessing for Cell Based Therapies’, edited by Dr Che Connon. (Wiley, 2016).
References
1. Mason C, Hoare M. Regenerative medicine bioprocessing: the need to learn from the experience of other fields. Regen. Med. 2006; 1(5): 615-23.
CrossRef
2. Chen VC, Ye J, Shukla P et al. Development of a scalable suspension culture for cardiac differentiation from human plu-ripotent stem cells. Stem Cell Res. 2015; 15: 365–75.
CrossRef
3. Le Blanc K, Frassoni F, Ball L et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a Phase II study. Lancet 2008; 371: 1579–86.
CrossRef
4. Masri F, Lawrence K, Wall I, Hoare M. An ultra scale-down methodology to characterize aspects of the response of human cells to processing by membrane separation operations. Biotech. Bioeng. 2017; 114(6): 1241–51.
CrossRef
5. Masri F. Uncovering the potential of ultra scale-down tools to enable cost-effective “Quality by Design”. Cell Gene Ther. Insights 2016; 2(4): 463–71.
CrossRef
6. Burguillos MA, Magnusson C, Nordin M et al. Microchannel acoustophoresis does not impact survival or function of mi-croglia, leukocytes or tumor cells. PLoS ONE 2013; 8:e64233.
CrossRef
7. Yang AH, Soh HT. Acoustophoretic sorting of viable mammalian cells in a microfluidic device. Anal. Chem. 2012; 849(24): 10756-62.
CrossRef
8. Cappon HJ, Stefanova LA, Keesman KJ. Concentration based flow control in acoustic separation of suspensions. Separation and Purification Technology 2013; 103: 321–7.
CrossRef
9. Becker FF, Wang XB, Huang Y, Pethig R, Vykoukal J, Gascoyne PR. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc. Natl Acad. Sci. USA 1995; 92: 860-4.
CrossRef
10. Valero A, Braschler T, Demierre N, Renaud P. A miniaturized continuous dielectrophoretic cell sorter and its applications. Biomicrofluidics, 2010. 4(2): p.022807.
CrossRef
11. Flanagan LA, Lu J, Wang L et al. Unique dielectric properties distinguish stem cells and their differentiated progeny. Stem Cells 2008; 26: 656-65.
CrossRef
12. Velugotla S, Pells S, Mjoseng HK et al. Dielectrophoresis based discrimination of human embryonic stem cells from differentiating derivatives. Biomicrofluidics 2012; 6(4): 44113.
CrossRef
13. Vahey MD, Voldman J. An equilibrium method for continuous-flow cell sorting using dielectrophoresis. Anal. Chem. 2008; 80(9): 3135-43.
CrossRef
14. Dholakia K, MacDonald MP, Zemánek P, Cizmár T. Cellular and colloidal separation using optical forces. Methods Cell Biol. 2007; 82: 467-95.
CrossRef
15. Chiou PY, Ohta AT, Wu MC. Massively parallel manipulation of single cells and microparticles using optical images. Na-ture 2005; 436: 370-72.
CrossRef
16. Imasaka T, Kawabata Y, Kaneta T, Ishidzu Y. Optical Chromatography. Anal. Chem. 1995; 67: 1763-65.
CrossRef
17. MacDonald MP, Spalding GC, Dholakia K. Microfluidic sorting in an optical lattice. Nature 2003; 426: 421-24.
CrossRef
18. Applegate R Jr, Squier J, Vestad T, Oakey J, Marr D. Optical trapping, manipulation, and sorting of cells and colloids in microfluidic systems with diode laser bars. Opt. Express 2004; 12(19): 4390-98.
CrossRef
19. Paterson L, Papagiakoumou E, Milne G et al. Passive optical separation within a ‘nondiffracting’ light beam. Biomed. Opt. 2007; 12(5): 054017.
CrossRef
20. Schouten HC, Kessinger A, Smith DM et al. Counterflow centrifugation apheresis for the collection of autologous periph-eral blood stem cells from patients with malignancies: a comparison with a standard centrifugation apheresis procedure. J. Clin. Apher. 1990; 5(3): 140-4.
CrossRef
21. Faradji A, Bohbot A, Schmitt-Goguel M et al. Apheresis-elutriation program for adoptive immunotherapy with autologous activated monocytes in cancer patients. Int. J. Artif. Organs 1991; 14(5): 304-12.
Webiste
22. Dlubek D, Dybko J, Wysoczanska B et al. Enrichment of normal progenitors in counter-flow centrifugal elutriation (CCE) fractions of fresh chronic myeloid leukemia leukapheresis products. Eur. J. Haematol. 2002; 68(5): 281-88.
CrossRef
23. Powell DJ Jr, Brennan AL, Zheng Z, Huynh H, Cotte J, Levine BL. Efficient clinical-scale enrichment of lymphocytes for use in adoptive immunotherapy using a modified counterflow centrifugal elutriation program. Cytotherapy 2009; 11(7): 923-35.
CrossRef
24. Tran CA, Torres-Coronado M, Gardner A et al. Optimized processing of growth factor mobilized peripheral blood CD34+ products by counterflow centrifugal elutriation. Stem Cells Trans. Med. 2012; 1: 422-29.
CrossRef
25. Coulais D, Panterne C, Fonteneau JF, Grégoire M. Purification of circulating plasmacytoid dendritic cells using counter-flow centrifugal elutriation and immunomagnetic beads. Cytotherapy 2012; 14(7): 887-96.
CrossRef
26. Sandgren P, Hild M, Sjödin A, Gulliksson H. Storage of Buffy-coat-derived platelets in additive solutions: in vitro effects on platelets prepared by the novel TACSI system and stored in plastic containers with different gas permeability. Vox Sang. 2010; 99(4): 341-7.
CrossRef
27. Lotens A, Najdovski T, Cellier N, Ernotte B, Lambermont M, Rapaille A. New approach to ‘top-and-bottom’ whole blood separation using the multiunit TACSI WB system: quality of blood components. Vox Sang. 2014 May 26 ePub.
DOI
28. Shimoda M, Itoh T, Iwahashi S et al. An effective purification method using large bottles for human pancreatic islet isolation. Islets 2012; 4(6): 398-404.
CrossRef
29. Huang LR, Cox EC, Austin RH, Sturm JC. Continuous particle separation through deterministic lateral displacement. Sci-ence 2004; 304: 987-90.
CrossRef
30. Davis JA, Inglis DW, Morton KJ et al. Deterministic hydrodynamics: taking blood apart. PNAS 2006; 103: 14779-84.
CrossRef
31. Zhang B, Green JV, Murthy SK, Radisic M. Label-free enrichment of functional cardiomyocytes using microfluidic deter-ministic lateral flow displacement. PLoS ONE 2012; 7: e37619.
CrossRef
32. Long BR, Heller M, Beech JP, Linke H, Bruus H, Tegenfeldt JO. Multi-directional sorting modes in deterministic lateral displacement devices. Physical Review E, 2008. 78(4): p. 046304.
33. Yamada M, Seki M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 2005; 5: 1233-9.
CrossRef
34. Yamada M, M1, Kano K, Tsuda Y et al. Microfluidic devices for size-dependent separation of liver cells. Biomed. Microde-vices 2007; 9: 637-45.
CrossRef
35. Nivedita N, Papautsky I. Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 2013; 7: 54101.
CrossRef
36. Bhagat AA, Kuntaegowdanahalli SS, Papautsky I. Continuous particle separation in spiral microchannels using Dean flows and differential migration. Lab Chip 2008; 8(11): 1906-14.
CrossRef
37. Kuntaegowdanahalli SS, Bhagat AA, Kumar G, Papautsky I. Inertial microfluidics for continuous particle separation in spi-ral microchannels. Lab Chip 2009; 9: 2973-80.
CrossRef
38. Hur SC, Henderson-MacLennan NK, McCabe ER, Di Carlo D. Deformability-based cell classification and enrichment us-ing inertial microfluidics. Lab Chip 2011; 11: 912–20.
CrossRef
39. Lee MG, Shin JH, Bae CY, Choi S, Park JK. Label-free cancer cell separation from human whole blood using inertial mi-crofluidics at low shear stress. Anal. Chem. 2013; 85(13): 6213-18.
CrossRef
40. Sim TS, Kwon K, Park JC, Lee JG, Jung HI. Multistage-multiorifice flow fractionation (MS-MOFF): continuous size-based separation of microspheres using multiple series of contraction/expansion microchannels. Lab Chip 2011; 11: 93-9.
CrossRef
41. Moon HS, Kwon K, Hyun KA et al. Continual collection and re-separation of circulating tumor cells from blood using multi-stage multi-orifice flow fractionation. Biomicrofluids 2013; 24: 14105.
CrossRef
42. Delahaye M, Lawrence K, Ward SJ, Hoare M. An Ultra Scale-Down Analysis of the Recovery by Dead-End Centrifugation of Human Cells for Therapy. Biotech. Bioeng. 2015; 112(5): 997–1011.
CrossRef
43. Tan SJ, Yobas L, Lee GY, Ong CN, Lim CT. Microdevice for the isolation and enumeration of cancer cells from blood. Bi-omed. Microdevices 2009; 11(4): 883-92.
CrossRef
44. Chen X, Da FC, Liu CC, Li H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sensors and Actuators B: Chemical, 2008; 130(1): p. 216-221.
45. Chen X, Cui D, Zhang L. Isolation of plasma from whole blood using a microfludic chip in a continuous cross-flow. Chin. Sci. Bull. 2009; 54: 324-27.
46. Zhang W, Kai K, Choi DS et al. Microfluidics separation reveals the stem-cell-like deformability of tumor-initiating cells. Proc. Natl Acad. Sci. USA 2012; 109(46): 18707.
CrossRef
47. Willoughby NA, Bock H, Hoeve MA et al. A scalable label-free approach to separate human pluripotent cells from differentiated derivatives. Biomicrofluidics 2016; 10(1):014107
48. Ji HM, Samper V, Chen Y, Heng CK, Lim TM, Yobas L. Silicon-based microfilters for whole blood cell separation. Bio-med. Microdevices 2008; 10(2): 251-7.
CrossRef
49. Cunha B. Peixoto C, Silva MM et al. Filtration methodologies for the clarification and concentration of human mesenchymal stem cells. J. Membr. Sci. 2015. 478; 117–29.
50. Cunha B, Silva RJ, Aguiar T et al. Improving washing strategies of human mesenchymal stem cells using negative mode ex-panded bed chromatography. Chromatogr. A 2016; 1429, 292–303.
CrossRef
Affiliations
Fernanda Masri1, Marieke A Hoeve2, Paul A De Sousa3 & Nicholas A Willoughby4
1 Sartorius Stedim Ltd., Longmead Business Centre, Surrey, UK.
2 MRC Centre for Regenerative Medicine, University of Edinburgh, UK.
3 Centre for Clinical and Brain Sciences, University of Edinburgh, UK.
4 Institute of Biological Chemistry, Biophysics and Bioengineering, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, UK.
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.