Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media
Cell Gene Therapy Insights 2017; 3(5), 345-358.
10.18609/cgti.2017.035
Cellular therapies are cell and tissue products sourced from biological materials that are employed as ‘living drugs.’ Such ‘living drugs’ require specialized biological support, namely biopreservation, to maintain optimal recovery, viability and return to function post-preservation. To achieve successful biopreservation, optimization of a multitude of parameters, including cooling/thawing rates based on the biophysics of specific cell types, the temperature of storage and transportation, as well as biopreservation media are of paramount importance. In particular, the choice of biopreservation media is pivotal for clearing regulatory hurdles and facilitating commercialization. Traditional extracellular-like (isotonic), home-brew cocktails (which may contain serum) may not be compatible with a Good Manufacturing Practices (GMP) clinical manufacturing process. This article will address the challenges associated with maintaining the viable recovery and functionality of ‘living drugs’, discuss the benefits and consequences of low-temperature biopreservation, outline Biopreservation Best Practices, and propose considerations for incorporating Biopreservation Best Practices into a GMP cell therapy product. Integration of Biopreservation Best Practices, beginning with the selection of optimized media, ensures that cells remain viable and functional throughout the cell product lifecycle, thereby optimizing manufacturing and clinical outcomes.
Average global life expectancy has accelerated by 5 years at the fastest growth rate since 1950 between 2000 and 2015, and now exceeds 71 years [1]. However, with this increase in lifespan comes a growing incidence of chronic human diseases prevalent in aging, such as cancer, cardiovascular and neurodegenerative diseases. As such, sustained progress in extending life expectancy and quality of life requires the continued development of novel therapies and approaches that address chronic human conditions prevalent in an aging population. A promising development in the treatment of chronic diseases is the use of living cells as ‘smart drugs’ that can effectively target specific tissues to rescue organ function or eradicate tumors. In the case of cancer, cell therapies have proven effective in patients who have exhausted other lines of standard care treatment, with reported results including complete remission and extension of median survival time [2,3]. As living drugs, cell therapies need to be viable and functional in order to be effective, and methods for preserving small molecules and monoclonal antibodies are not similarly effective for cell-based products. Rather, they require cellular support during all phases of the product lifecycle, from source material acquisition to final delivery and patient administration. Optimized strategies to maintain the viable recovery and efficacy of cell therapies during all stages of collection, manufacturing and delivery are essential to realize the potential of these promising medical advances (Figure 1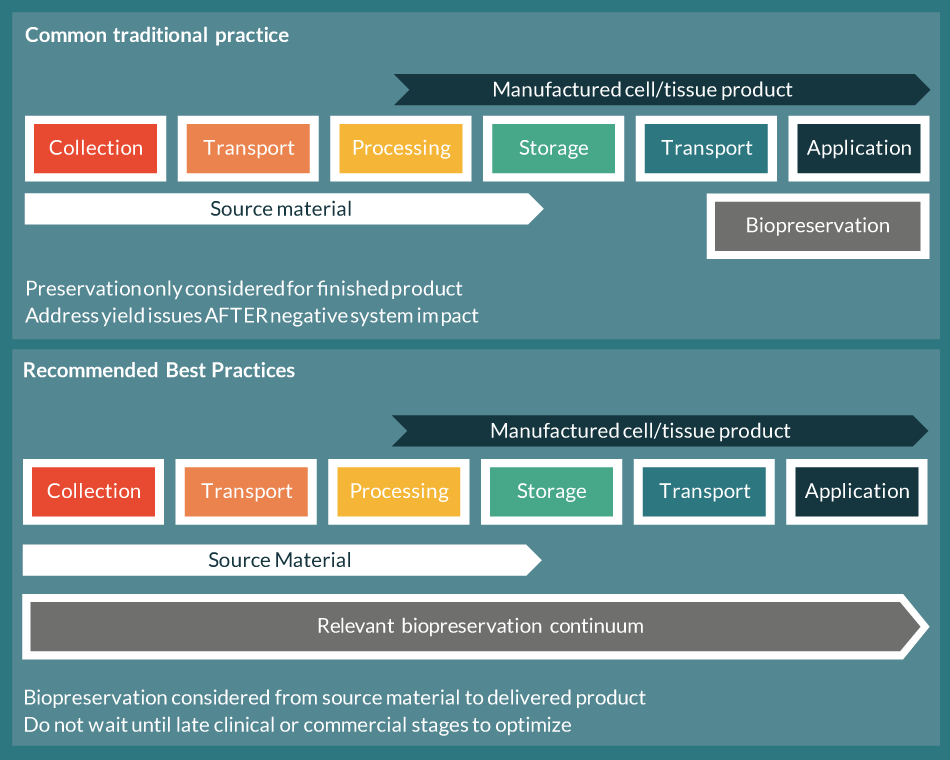 Biopreservation practices.Common traditional biopreservation methods focus only on the finished product. Biopreservation Best Practices recommends optimizing biopreservation and reducing stability stresses from collection of source material through final cell/tissue product application. ).
Biopreservation practices.Common traditional biopreservation methods focus only on the finished product. Biopreservation Best Practices recommends optimizing biopreservation and reducing stability stresses from collection of source material through final cell/tissue product application. ).
The critical focus of biopreservation, or product stability, is reflected in the broad topics and expertise that are reported as perspectives within this spotlight issue. Rafiq and Coopman describe considerations of biopreservation that emerge with manufacturing scale-up. Stacey speaks to standards and quality attributes for cryopreservation of stem cells, which are more difficult to define than in normothermic cell models and have proven variable and more complex within biopreservation cell models. Fuller provides cryobiology principles to allow further understanding into the complex biophysical considerations at low temperatures. This collection of expert perspectives regarding biopreservation, and related considerations to the scientific background, Good Manufacturing Practices (GMP), Quality/Regulatory attributes, and translation to clinical applications, is a unique and valuable resource for clinical and commercial developers of cellular therapies and regenerative medicine applications.
The challenge of cell therapy commercialization
Cell therapies originate from biological sources, such as tissue biopsies, blood and bone marrow. This biological seed material is then manipulated in a laboratory or manufacturing facility to develop the cell therapy product. Whether autologous or allogeneic, the development and eventual introduction of the therapeutic dose into a patient involves rounds of transportation between the collection site and the laboratory or cell manufacturing facility, as well as storage periods of varying durations for logistic arrangements and quality control checks. Unlike pharmaceutical or small molecule agents, cells and tissues have unique requirements in order to remain viable and functional while outside the body or culture conditions. Therefore, an essential component of the cell manufacturing lifecycle is to preserve cells during handling and storage. More so, improper preservation of biologic specimens at any step in the process may negatively impact all subsequent manufacturing steps, and may adversely impact the final cell therapy product.
During the early developmental phases of cell therapies, the storage and handling of cells is often not a critical step in the product lifecycle. In many cases, the sites of collection and processing may be physically conjoined or in close proximity, ensuring cellular products can be transported and manipulated with little impact to stability. As preclinical product development progresses, the process typically requires the need for more operators and resources, and may require additional collection or processing sites. Cell handling and transportation to remote sites therefore becomes more complex with increased risk potential. In a large-scale clinical trial, the collection and processing of hundreds of patients may be spread between remote sites. At this stage, an optimized means to store and transport cell-based products between remote collection and manufacturing sites becomes more critical for minimizing variability, increasing clinical efficacy and ensuring commercial viability.
Low-temperature biopreservation considerations: hypothermic & cryogenic conditions
As soon as biological specimens are removed from the body and normothermic conditions, deleterious environmental stresses begin to degrade the source material. From a clinical efficacy standpoint, these environmental stresses are compounded at each step of the cell product lifecycle, introducing sample variability and the potential for impaired therapeutic function, which may even lead to clinical inefficacy and termination of a potentially effective treatment. From a financial standpoint, loss of cell yield, viability and function adds significant cost to cell therapies by increasing the potential for repeat sampling or additional processing steps to expand cell numbers or resuscitate function [4]. Biopreservation refers to the processes required to maintain the health and function of biologics outside the body, as well as suppress the degradation of these biological materials to ensure a return to function post-preservation [5]. By reducing the stresses experienced by cells and tissues ex vivo, optimized biopreservation processes can extend the time that cells and tissues remain outside of normothermic conditions. For commercial cell therapy products, effective biopreservation protocols provide flexibility in manufacturing and shipping, facilitate effective process development, and reduce manufacturing costs. An ideal biopreservation protocol would maintain biological function throughout the product lifecycle, providing ‘vein-to-vein’/‘needle-to-needle’ support from the time the sample is collected from the donor to the time it is administered to the recipient.
Under normothermic conditions, cells must continually generate energy in the form of ATP to maintain the intracellular environment and permit essential enzymatic reactions. Indeed, approximately 20% of energy production is used to drive selective ion pumps that maintain the intracellular ionic balance and resting membrane potential [6], a figure that can rise to approximately 66% in metabolically active neural tissue [7]. Cells specifically regulate the transport of sodium (Na+), potassium (K+) and calcium (Ca2+) ions across the plasma membrane, store additional Ca2+ ions within the endoplasmic reticulum, and effectively sequester K+ within the cytoplasmic compartment. The energy required to maintain these ion gradients is generated within the cell by mitochondria, which convert glucose and other substrates into ATP through an oxygen dependent process. Reactive oxygen species are a natural byproduct of energy production, but are effectively countered by cellular antioxidant mechanisms of defense [8]. Maintenance of cellular metabolism outside the body or dedicated cell culture facilities under normothermic conditions would be impractical, and would require a continual oxygen supply, sufficient nutrients to maintain metabolism, and a means to remove waste products. In contrast to normothermia, each 10°C decrease in temperature reduces metabolism approximately 50% for energetically active tissue such as the brain [9], minimizing the environmental requirements of cells outside culture conditions. This Q10 coefficient reduction in metabolism limits the need for oxygen and substrates, minimizes waste production, preserves cellular ATP levels and attenuates the molecular processes that contribute to ischemic injury both during storage and upon a return to normothermia post-preservation. As a result, low-temperature biopreservation, both at 2–8°C hypothermic temperatures, or cryogenically frozen between -80°C and -196°C, is the most common and preferred method of storage employed in cell therapy and regenerative medicine applications.
Despite the metabolic benefits of low-temperature biopreservation, temperature reduction exerts unique stresses that must be carefully addressed to maximize cell viability and function upon a return to normothermia. Cells at reduced temperatures (hypothermic storage and cryopreservation) undergo reduced membrane ion pump activity and a physical reorganization of the plasma membrane that increases permeability [10]. Consequentially, Na+ ions flow into the cell and K+ ions escape into the extracellular compartment [11]. The Ca2+ concentration inside the cell also rises over 1000-fold due to both a release of Ca2+ from the endoplasmic reticulum stores, and an influx of Ca2+ from the extracellular environment [12]. Under normothermic conditions, this flow of ions across the plasma membrane would be controlled by ATP-dependent membrane pumps. However, low temperatures also slow mitochondrial metabolism and facilitate the depletion of the cellular ATP levels necessary to fuel these ATP-dependent membrane pumps. In addition, impaired mitochondrial function results in the increased generation of damaging oxygen free radicals that may exceed the cell antioxidant scavenging capacity [13]. Although a small amount of energy can be generated anaerobically extra-mitochondrially by the breakdown of glucose via glycolysis, the resultant formation of lactic acid lowers pH and triggers further cellular damage [11]. Unregulated ion movement, combined with slowed membrane pumps, results in interruption of the delicate intracellular ionic balance as well as osmotic cell swelling. Further reduction in temperature to below the freezing point can induce the formation of intracellular ice crystals that can physically damage cells further by puncturing membranes and disrupting intracellular structures. More importantly, growth of intracellular and extracellular ice crystals during freezing results in continued concentration of salt ions and shifting pH and salinity, that adversely, and sometimes irreversibly, impact intracellular and membrane proteins. Such cold-induced stresses, combined with a reduced ability to scavenge free radicals, can accumulate to levels that induce cell death. While on the surface hypothermic and frozen storage may appear to impart divergent stresses, the transition to, through, and from the frozen state exerts hypothermic stresses on cells. Indeed, cells can experience profound supercooling (i.e. non-frozen temperatures below the freezing point of a solution) of between 10 and 20°C, depending on the freezing rate, before the advent of ice formation [14]. Furthermore, the non-frozen fraction during cryopreservation experiences a hypothermic continuum until reaching the vitrified state below the glass transition temperature. As such, hypothermic stresses are ever-present throughout the hypothermic continuum regardless of whether the sample is in the frozen or non-frozen state, and can contribute to the damage observed following cryopreservation [15].
Even cells that do not lyse during hypothermic/cryogenic temperature exposure are sometimes irreversibly damaged after a return to normothermic temperatures. A certain percentage of cells are damaged to the point that they will perish over time – by necrosis, programmed apoptosis and secondary necrotic cell death – in a process known as Delayed Onset Cell Death [16,17]. Indeed, Stroncek et al. reported that transduced peripheral blood lymphocytes apparently viable post-preservation exhibit a decline in survival over several days in culture, although at the time the potential link to Delayed Onset Cell Death was not established [18]. While cell loss can be mitigated by additional culture in the laboratory or cell manufacturing facility [19], expanded post-preservation culture would necessitate remote cell culture facilities at the clinic and a delay in patient administration, both of which add significant financial costs and could render the cell therapy non-viable from a commercial standpoint. In addition, cells that survive have likely undergone population selection, potential genetic drift and may exhibit adaptations that negatively influence downstream cellular function in clinical applications. Ultimately, after low temperature biopreservation, the ordered priorities of the cell are survival, repair and recovery, and then functional return. This is relevant in the context of cell therapy products, which are expected to function upon thaw and delivery to the patient.
Optimized biopreservation methods improve cell viability & functionality
While the biopreservation of cell therapy products appears daunting, many of the stresses imposed by low temperature biopreservation can be mitigated by optimizing methods and storage media. In practice, there are numerous stress points that occur during the workflow (Figure 1), and transition to and from low temperature biopreservation at both hypothermic and frozen conditions. For hypothermic biopreservation, critical steps include the choice of storage media at relevant stages, the rate and temperature of solution addition, the storage temperature, the warming rate and, ultimately, the removal or dilution of the storage solution. As opposed to hypothermic storage, cryopreservation imparts additional stress points related to ice formation and includes, in sequential order, the selection of an appropriate biopreservation solution and cryoprotectant, the rate of cryoprotectant addition, the temperature of cryoprotectant addition, the temperature of ice nucleation, cooling rate, storage temperature, warming rate, and finally, the removal or dilution of the cryoprotectant and storage medium [20]. In addition, within a cell/tissue manufacturing process, the variability of source material quality (apheresis/leukapheresis/tissue) and the collection/transition of cells from expansion/processing to final formulation/fill are increasingly recognized as potential bottleneck stress points in the overall workflow. Each of these stress points impacts post-preservation viability and functionality, and is deserving of detailed examination. However, for the purposes of this review, the focus on biopreservation media was chosen for further discussion as it constitutes an early and critical step in both hypothermic and cryogenic storage applications (Figure 2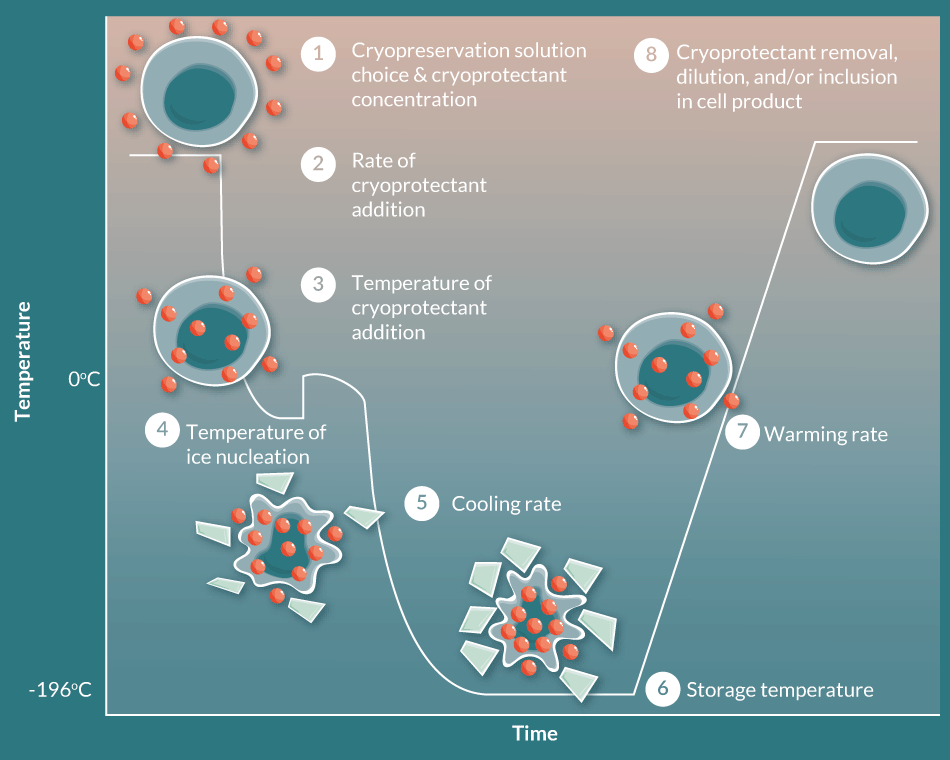 Critical steps in the cryopreservation process. Even within the singular cell manufacturing step of cryopreservation, there are multiple points for Risk, and subsequently multiple points for Optimization in consideration to Biopreservation Best Practices. Modified from: Acker JP; Biopreservation and cellular therapies. ISCT Telegraft15(2), 8–11 (2008)).
Critical steps in the cryopreservation process. Even within the singular cell manufacturing step of cryopreservation, there are multiple points for Risk, and subsequently multiple points for Optimization in consideration to Biopreservation Best Practices. Modified from: Acker JP; Biopreservation and cellular therapies. ISCT Telegraft15(2), 8–11 (2008)).
The first consideration for an optimized biopreservation solution is to address the problems associated with the ionic imbalance. Rather than utilizing an isotonic, extracellular-like basal media composed of an ionic balance designed for normothermic cell growth, wash or infusion, cells stored at low temperatures are recommended to be balanced in an ionic composition similar to that of the intracellular milieu. Doing so will reduce concentration gradients and uncontrolled ion flux across the cell membrane and will compensate for the reduced activity of the dysfunctional and ATP-depleted membrane pumps. Further addition of polysaccharides and other large cell impermeant molecules increases extracellular osmolality and reduces subsequent osmotic swelling and plasma membrane damage. A hypertonic solution also facilitates more rapid cellular dehydration that decreases the probability of intracellular ice formation during freezing, and would allow greater flexibility with freezing rate. An optimized biopreservation media is also recommended to contain appropriate antioxidants that minimize the impact of damaging free radicals generated by the mitochondria, as well as energetic precursors to speed ATP generation upon returning to normothermia. Optimized biopreservation media are also recommended to contain pH buffers that have a low degree of temperature sensitivity to maintain appropriate pH buffering capacity during hypothermic conditions, as well as a robust capacity to stabilize the reduction in pH associated with the cellular production of lactate.
The misconception among many who deal with cryopreservation is that cryoprotective agents (CPAs) are needed to minimize ice formation because it is the ice that kills the cells. While the CPA does contribute to reduced intracellular ice formation, an additional way a CPA protects cells is by minimizing a significant increase in ions and salt concentration in the solution during freezing. In fact, the cellular toxicity from increased concentration of salts is shown to be more detrimental to cell viability than the CPA, in what is known to cryobiologists as ‘the solution effects’ [21]. Dimethylsulfoxide (DMSO) is a membrane-permeating cryoprotectant that has been used for hematopoietic stem cell transplants in patients for decades. And while early reports correlate DMSO infusion with serious adverse events, most side effects include treatable symptoms such as nausea, vomiting, headache and cough consistent with low-grade anaphylaxis [22,23]. More recent studies indicate that serious adverse events may not be a result of DMSO per se, but rather by the white blood cell/granulocyte concentration of the infused product [24]. Cells frozen in a novel intracellular-like DMSO-containing cryopreservation solution as part of a pediatric cell therapy animal model have also been safely injected directly into the hearts of neonatal pigs, utilizing the DMSO-containing intracellular-like cryopreservation media as an excipient without wash [25]. With a well-documented clinical history, and accepted hypersensitivities for use in multiple administration models, DMSO continues to be the most widely-accepted, clinically tracked, and effective permeating cryoprotectant used in cell therapy products to date. For cryogenic storage, optimized biopreservation may include permeating cryoprotectants such as DMSO to minimize cellular injury due to solution effects, and it would be recommended to also include multiple cryoprotectants that act in both permeating and non-permeating mechanisms. As such, the potential for DMSO toxicity may be minimized by the concurrent use of additional cryoprotectants that act in a non-permeating mechanism. Optimized cryomedia, therefore, would be designed to engineer an effective balance of both permeating and non-permeating cryoprotectants to effectively reduce the toxicity of any single cryoprotectant (such as DMSO). Alternative cryoprotectant options, as well as methods modifications, are available to clinical manufacturers and patient populations sensitive to the potential clinical effects of DMSO; and the risk:reward versus development burden considerations are ongoing and worthy discussions in the regenerative medicine field. Utilization of an optimized biopreservation media is an early and critical step to designing an optimized biopreservation protocol, and can potentially improve viable cell recovery post-preservation, and accelerate the functional return (i.e., potency) that is increasingly recognized as a critical parameter for cellular therapy clinical success.
Biopreservation of commercial cell therapy products
Despite their clinical and commercial promise, novel cellular therapies are nascent technologies that have not yet achieved widespread clinical utility and commercial viability. Since the US Food & Drug Administration (FDA) approved Carticel as the first cellular therapy in 1997, only a handful of additional products have obtained marketing authorizations worldwide. The largest barrier to entry for commercial viability involves the relative cost–effectiveness of cell therapy products in comparison to conventional pharmaceutical and biopharmaceutical treatments [26]. Indeed, cell manufacturing is currently substantially more expensive than traditional pharmacologic and small molecule products due to the need for more complex and specialized reagents, instrumentation and processes. Followed closely behind cost are issues related to product efficacy, reimbursement, safety, regulations and infrastructure [26]. Within all of these barriers, an important and often overlooked element is product stability and shelf-life where biopreservation comes into effect. For example, when approved by the FDA in 2010, Dendreon’s Provenge® (sipuleucel-T) dendritic cellular immunotherapy adopted a manufacturing model with short stability of the apheresis starting material, and limited 18-hour non-frozen shelf-life of the finished product. This 18-hour product stability window necessitated a complex and time-critical manufacturing and supply chain with high cost-of-goods and product pricing, which has been argued was a major factor in Provenge’s limited market adoption [27]. Cell therapy companies have since learned the lessons of the Provenge model, and are beginning to standardize product development with an eye towards increased product stability at all relevant points in the needle-to-needle workflow. In one noted next generation example, Kite Pharma has increased the stability of its lead oncology product axacabtagene ciloleucel from 18 hours to 2 weeks by transitioning to a cryopreserved product [28], reducing manufacturing constraints and providing logistical flexibility at reduced overall cost. In practice, a lack of standardization and guidelines necessitates that each novel cell therapy developer validate biopreservation protocols for each product, and the varying biopreservation methods may result in cumulative variability in efficacy, costs, risks and Quality/Regulatory footprint. The standardization of biopreservation media and methods (i.e., Biopreservation Best Practices) may increase the efficiency of regenerative medicine clinical development and potential commercialization, and provide a more effective roadmap for optimizing methods for each cell product.
What constitutes Biopreservation Best Practices for cell therapy and regenerative medicine products? With regards to an early step in the biopreservation continuum, the selection of appropriate media, many non-frozen and freezing methods employ extracellular-like ‘home-brew’ cocktails based on traditional formulations, which often are minimally effective for research and development, but are inefficient, increase risk and are not scalable for GMP clinical manufacturing. Historical home-brew cocktails may not be manufactured according to using USP-grade materials (or multicompendial/highest quality), which may result in Batch-to-Batch (or Lot-to-Lot) variability. By contrast, Biopreservation Best Practices recommendations for an optimized media would support intracellular-like biopreservation media formulation specifically designed to mitigate the detrimental effects of cold temperature storage [20,29,30]. Such intracellular-like media is recommended to be manufactured according to GMP in a certified facility (ISO, GMP, Regulatory body), with an appropriate Quality and Regulatory footprint and packaging options amenable to closed manufacturing systems. Recommended biopreservation media would also be free of animal and human serum and proteins to reduce the potential for disease transmission, as well as simplify the Quality/Regulatory risk assessment step. Elimination of serum in particular, not only reduces the risks and costs associated with the use of properly vetted animal/human-derived products, but also facilitates the GMP production by reducing the inherent Batch-to-Batch variability of serum which have been recognized and is almost impossible to characterize. The components of the media and the protocol methods also require consideration as part of an overall Quality/Regulatory/Safety footprint, in order to appropriately qualify within a GMP clinical application, and more so should the biopreservation media be considered as an excipient within the final cell product. The biopreservation media is recommended to have a risk profile that would allow consideration for qualification as an excipient for direct delivery to patients, and have clinical supporting information to support the related clinical risk assessment (Figure 3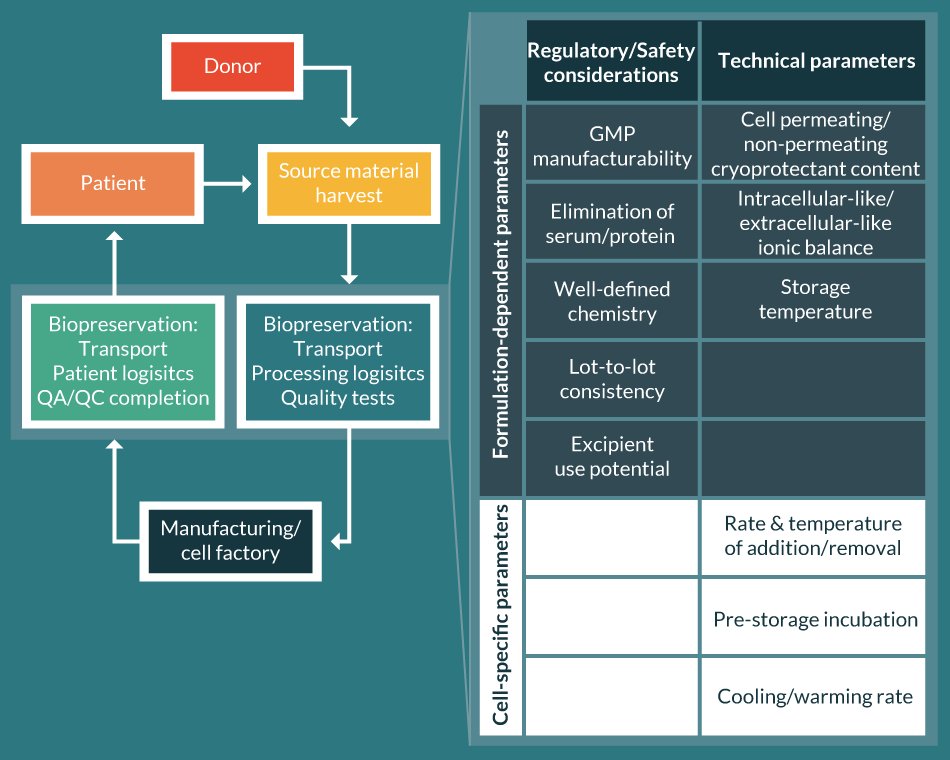 Parametric considerations for Biopreservation Best Practices.Selection of the right biopreservation formulation can simultaneously address multiple regulatory and technical considerations (shaded within the table on the right) and speed the path to commercialization. ).
Parametric considerations for Biopreservation Best Practices.Selection of the right biopreservation formulation can simultaneously address multiple regulatory and technical considerations (shaded within the table on the right) and speed the path to commercialization. ).
Historical biopreservation formulations can still be compounded in-house by using traceable raw materials of the highest available quality (USP or multicompendial/highest quality), but would likely not be manufactured according to GMP with appropriate per batch Quality Control release assays or stability studies, and should undergo an independent internal risk assessment for safety and stability. In lieu of in-house home-brews, commercially available alternatives that adhere to Biopreservation Best Practices recommendations are available and have been cited in a number of regenerative medicine clinically relevant publications. Examples of such media include the intracellular-like formulations HypoThermosol FRS and CryoStor. In addition, Biopreservation Best Practices recommendations would qualify methods/protocols optimization addressing each point of stability risk (Figure 1), similar to a Failure Modes and Effects Analysis (FMEA), throughout the cell/tissue lifecycle, as well as within each biopreservation methods step (Figure 2). These methods optimization steps may include, but are not limited to: collection and transport of blood/tissue/marrow, intermediate hold steps and cell banks, final cell product biopreservation and packaging, temperature-controlled shipping and monitoring, post-preservation processing, and patient administration. An emerging consideration within the development of regenerative medicine cell-based products is the aspect of Biologistics – the processes, tools and data used to manage and monitor the movement of biologic materials across time and space. This report does not address the aspects of Biologistics Best Practices; however O’Donnell and colleagues have comprehensively described these considerations previously [31-33]. Cumulatively, the multiple points of potential risk and stress related to the workflow from collection, through manufacturing and supply chain logistics, to patient administration, are all inter-related with Biopreservation and Biologistics Best Practices.
Summary
The use of manufactured cells as ‘living drugs’ to combat chronic disease promises to dramatically improve human health and increase lifespan. However, advanced cellular therapies require specialized environmental support in order to maintain effectiveness throughout the product lifecycle. Biopreservation refers to all the practices required to minimize cell death and damage ex vivo, as well as facilitate a return to function post-preservation. Whether low temperature biopreservation is intended for hypothermic or cryogenic applications, there are key steps in the process that require optimization for each cell type, including (but not limited to): the selection of appropriate hypothermic/cryogenic biopreservation solutions and cryoprotectants, the rate of media addition, the temperature of media addition, the temperature of ice nucleation, cooling rate, storage temperature, warming rate, and the removal or dilution of the storage media and cryoprotectants. Careful consideration of each of these steps can greatly improve biopreservation efficacy. An early critical step in any biopreservation strategy is the selection of appropriate storage media, and is the primary focus of this review. Biopreservation Best Practices with regards to media selection is recommended to consider GMP-manufactured solutions composed of the highest quality raw materials, with an appropriate Quality/Regulatory footprint that facilitates integration into cell therapy and regenerative medicine applications. Optimized biopreservation media would ideally be formulated with an intracellular-like ionic balance devoid of pathogenic animal and human proteins, and be safe for consideration as an excipient with appropriate qualification. In reality, the adoption of Biopreservation Best Practices minimizes the stresses associated with collection, manufacturing, storage, and transport, improves viable and functional cell recovery following biopreservation, and ensures that cells maintain their targeted therapeutic efficacy through the product lifecycle. In doing so, Biopreservation Best Practices reduces manufacturing costs, provides logistical flexibility, and ensures therapeutic potency at the clinic. Only by delivering the best possible treatments to patients can the true potential of regenerative medicine be fully realized, and only by manufacturing these cell/tissue products to be commercially and clinically viable can these promising therapies be sustainable over the long-term to benefit the greater global population.
Financial and competing interests disclosure
BJH, AA, and AJM are employees of BioLife Solutions, Inc. BJH and AA are Senior Application Scientists. AJM is Senior Vice President & Chief Technology Officer. No writing assistance was utilized in the production of this manuscript.
References
1. World Health Organization. WORLD HEALTH STATISTICS – MONITORING HEALTH FOR THE SDGs. World Health Organization. 2016.
2. Maude SL, Frey N, Shaw PA et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014; 371(16): 1507–17.
CrossRef
3. Kochenderfer JN, Somerville RPT, Lu T et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels
DOI
4. Baust JG. Concepts in Biopreservation. Advances in Biopreservation. 2006; 1–14:
Website
5. Snyder KK, Van Buskirk RG, Mathew AJ, Baust JG, Baust JM. Biological Packaging for the Gobal Cell and Tissue Therapy Markets. Bioprocess J. 2004; 3(3): 39–45.
CrossRef
6. Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997; 77(3): 731–58.
Website
7. Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012; 32(7): 1222–32.
CrossRef
8. Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009; 417(1): 1–13.
CrossRef
9. Fuhrman GJ, Fuhrman F. Oxygen consumption of animals and tissues as a function of temperature. J. Gen. Physiol. 1959; 42(4): 715–22.
CrossRef
10. Stefanovich P, Ezzell RM, Sheehan SJ, Tompkins RG, Yarmush ML, Toner M. Effects of hypothermia on the function, membrane integrity, and cytoskeletal structure of hepatocytes. Cryobiology 1995; 32(4): 389–403.
CrossRef
11. Boutilier RG. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001; 204(Pt 18): 3171–81.
Website
12. Ivanov KP. Restoration of vital activity of cooled animals without rewarming the body. Eur. J. Appl. Physiol. 2009; 105: 5–12.
CrossRef
13. Jarmuszkiewicz W, Woyda-Ploszczyca A, Koziel A, Majerczak J, Zoladz JA. Temperature controls oxidative phosphorylation and reactive oxygen species production through uncoupling in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2015; 83: 12–20.
CrossRef
14. Prickett RC, Marquez-Curtis LA, Elliott JAW, McGann LE. Effect of supercooling and cell volume on intracellular ice formation. Cryobiology 2015; 70(2): 156–63.
CrossRef
15. Baust JG, Snyder KK, Van Buskirk R, Baust JM. Integrating Molecular Control to Improve Cryopreservation Outcome. Biopreserv. Biobank 2017; 15(2)
DOI
16. Baust JM. Molecular mechanisms of cellular demise associated with cryopreservation failure. Cell Preserv. Technol. 2004; 1(1): 17–31.
CrossRef
17. Van Buskirk RG, Snyder KK, Mathew AJ, Baust JG, Baust JM. Navigating the postpreservation viability fog. Genet. Eng. Biotechnol. News. 2006; 26(19).
Webiste
18. Stroncek DF, Hubel A, Shankar RA et al. Retroviral transduction and expansion of peripheral blood lymphocytes for the treatment of mucopolysaccharidosis type II, Hunter’s syndrome. Transfusion 1999; 39(4): 343–50.
CrossRef
19. Otsuru S, Hofmann TJ, Raman P et al. Genomic and functional comparison of mesenchymal stromal cells prepared using two isolation methods. Cytotherapy 2015; 17(3): 262–70.
CrossRef
20. Creer MH, Lemas MV, Mathew AJ. Practical handbook of cellular therapy cryopreservation. AABB Press 2015. 176.
21. Mazur P. Freezing of living cells: mechanisms and implications. Am. J. Physiol. Cell Physiol. 1984; 247(3): C125–42.
Webiste
22. Stroncek DF, Fautsch SK, Lasky LC, Hurd DD, Ramsay NK, McCullough J. Adverse reactions in patients transfused with cryopreserved marrow. Transfusion 1991; 31(6): 521–6.
CrossRef
23. Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood 1990; 75(3): 781–6.
Webiste
24. Truong TH, Moorjani R, Dewey D, Guilcher GMT, Prokopishyn NL, Lewis VA. Adverse reactions during stem cell infusion in children treated with autologous and allogeneic stem cell transplantation. Bone Marrow Transplant 2016; 51(5): 680–6.
CrossRef
25. Peral S, Burkhart H, Oomimen S et al. Safety and Feasibility for Pediatric Cardiac Regeneration Using Epicardial Delivery of Autologous Umbilical Cord Blood-Derived Mononuclear Cells Established in a Porcine Model System. Stem Cells Transl. Med. 2015; 4: 195–206.
CrossRef
26. Davies BM, Rikabi S, French A et al. Quantitative assessment of barriers to the clinical development and adoption of cellular therapies: A pilot study. J. Tissue Eng. 2014; 5: 2041731414551764.
CrossRef
27. Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015; 370(1680)
DOI
28. Locke FL, Neelapu SS, Bartlett NL et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol. Ther. 2017; 25(1): 285–95.
CrossRef
29. Mathew AJ. A review of cellular biopreservation considerations during hair transplantation. Hair Transpl. Forum Int. 2013; 23(1): 6–11.
Webiste
30. Pollock K, Yu G, Moller-Trane R et al. Combinations of Osmolytes, Including Monosaccharides, Disaccharides, and Sugar Alcohols Act in Concert During Cryopreservation to Improve Mesenchymal Stromal Cell Survival. Tissue Eng. Part C Methods 2016; 22(11): 999–1008.
CrossRef
31. O’Donnell K. Web connected biologistics: cold chain lessons learned from the pharmaceutical industry and how SMART shippers have come of age
Webiste
32. O’Donnell K. Moving from passive to rescue design packaging: helping cells arrive alive with smart shippers. Cell Gene Ther. Insights 2015; 1(2): 163–71.
CrossRef
33. Abazari A, Hawkins BJ, Fink J, O’Donnell K, Mathew AJ. Next generation technology, procedures, and products facilitate biopreservation best practices for cellular therapies
Webiste
Affiliations
Brian J Hawkins, Alireza Abazari & Aby J Mathew
BioLife Solutions, Inc.,
Bothell, WA, USA
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.