The regulation of CAR-T cells
Cell Gene Therapy Insights 2017; 3(4), 239-253.
10.18609/cgti.2017.030
The clinical success of genetically modified T cells utilizing chimeric antigen receptors has been seen in the treatment especially of B cell hematological malignancies in several clinical trials to date. The regulatory requirements for CAR-T cell therapy is a challenging task because of the unique and novel nature of each therapy. The manufacture of genetically modified T cells, whether they are derived from an autologous or allogeneic source, requires reproducibility, safety and efficacy of the final product. Therefore, the regulatory approach taken for these cell therapies is dictated not only by the manufacture of the products but also by their intended clinical use and method of clinical delivery.
Chimeric antigen receptors (CARs) are genetically engineered to combine an extracellular single-chain antigen-recognition domain (ScFv, usually derived from a specific murine-derived monoclonal antibody) with one or more intracellular T cell signaling domains. Gene transfer techniques have allowed CARs to be introduced into normal T cells and thereby, redirecting the cells to target specific new antigens, which is independent of major histocompatibility complexes [1,2]. T cells are usually transduced with CARs that are encoded in lentiviral or retroviral vectors, although other methods such as electroporation and RNA-based methods can also be employed [3,4]. These integrating vectors allow for the permanent modification of the genome and increase the potential for long lasting expression of the CAR protein for the life of the T cell. The components and structure of the CAR signaling domain are critical for maximal activation, expansion and persistence of CAR-T cells. The so-called ‘first-generation’ CARs, which included only the antigen-recognition domain with an intracellular CD3ζ signaling domain, had limited clinical activity [3,5,6]. Several groups have worked to improve treatment efficacy by developing ‘second-generation’ CARs, which include a costimulatory domain typically derived from CD28 or CD137 (also known as 4-1BB), in addition to the CD3ζ domain.
It has recently been shown in several clinical trials that genetically modified T cells expressing CARs that target the CD19 antigen on the cell surface of tumor cells have significantly treated B cell hematological malignancies [7], including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma (NHL). Most of these clinical trials were conducted in the autologous setting, whereby T cells are procured from an apheresis product collected from the patient to be treated. Although in a therapy utilizing T cells expressing ErbB-targeted CAR and an IL-4 responsive chimeric cytokine receptor, the starting material consisted of peripheral blood mononuclear cells (PBMCs) isolated from patient-derived whole blood [8]. The patient’s cells are then genetically modified using an attenuated retroviral vector (usually a γ-retrovirus or self-inactivating lentivirus) expressing the CAR along with possibly other genes of interest, such as suicide-triggering or cytokine-expressing genes [9], and then expanded to several billions of cells. These cells are usually cryopreserved and then reinfused into the patient at specific dosages, which are usually determined as the number of CAR+ T cells/kg body weight.
Recently, investigators at Great Ormond Street Hospital and University College London’s Institute of Child Health have treated two infants with relapsed/refractory ALL, with a single dose of off-the-shelf CD19 CAR+ TCR– CD52– allogeneic T cells derived from a healthy donor (UCART19) [10]. The manufacture of allogeneic CAR-specific T cell therapies follow more or less similar manufacturing processes with respect to the transduction and expansion stages described above for autologous CAR-T cells except that the manufacturing starts with an apheresis product originating from a single donor so as to provide treatments to large numbers of patients. For most of the allogeneic CAR-T cell products, the genetically modified T cells are further genetically engineered usually by gene editing to minimize graft versus host disease (GvHD) since studies have clearly demonstrated that allogeneic CAR-T cells can not only induce tumor regression but also drive GvHD [11,12]. In this instance, the UCART19 infused allogeneic CARs expressing T cells were gene edited by nucleases to disrupt expression of the endogenous T cell receptors (TCRs) to avoid alloreactivity [13].
The nature of a medical condition may dictate whether autologous or allogeneic therapy is most appropriate. For instance, allogeneic therapy may be the only option when emergency care is required because of the time needed to produce autologous therapy. Because of the potential for causing GvHD, the use of donor-derived cells, especially en route to off-the-shelf T cells, needs to be further evaluated. There are currently many studies that use multiple techniques to address safety concerns to avoid GvHD. However, there are many diseases where both autologous and allogeneic therapies are being considered.
From a regulatory standpoint, when cell therapies first evolved, the US Food and Drug Administration (FDA) struggled to regulate them since at that time regulations were tailored to different types of drugs; small molecule therapeutics that were usually chemical in nature. The regulations on cell therapy products have evolved over time and have become relatively clearer.
Since the manufacture of CAR-specific T cells, as briefly described above, requires what we call ‘more than minimal manipulations’ (MTMM), the cell product is clearly regulated under the Public Health Service Act (PHSAct) section 351. Regulating the CAR-T cell product under section 351 is a key distinction because section 351 requires pre-market approval usually via the clinical trial pathway. To prevent failure at marketing authorization approval, the manufacturing, pre-clinical and Phase 1 trial considerations have to carefully navigate the regulatory environment.
The FDA has made substantial progress in its efforts to address the rapidly evolving technology equated with the manufacture of CAR specific T-cell therapies. This is evidenced by the numerous guidance documents on cell and gene therapies that have been generated in the past decade as depicted in Box 1. The agency is a partner at the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). In the field of cell and gene therapy, several ICH Guidelines (Box 1) were developed so as to achieve harmonization via a process of scientific consensus with regulatory and industry experts working side by side.
| Box 1: Regulatory guidelines related to cell and gene therapies |
|---|
FDA [56]
Q5A (R1): Viral Safety evaluation of Biotechnology Products Derived from Cell lines of Human or animal origin Q5B: Quality of Biotechnological Products: analysis of the expression Construct Q5D: Cells used for Production of r-DNA Derived Protein Products Derivation and Characterization of Cell Substrates Used for Production of Biotechnological/Biological Products Q6B: Specifications — test Procedures and acceptance Criteria for Biotechnological/ Biological Products Q7: Good manufacturing Practice Guide for active Pharmaceutical ingredients Q8 (R2): Pharmaceutical Development Q9: Quality risk management Q11 (Draft): Development and manufacture of Drug Substances (Chemical entities and Biotechnological/Biological entities), current step 2 version dated 19 may 2011 |
Manufacturing
There are currently many challenges facing the CAR-T therapy industry particularly in the regulated manufacture of these products under current Good Manufacturing Practices (cGMP) [14]. cGMPs are described in the Code of Federal Regulations (CFR) Parts 210 (Definitions), 211 (Basic Instructions) and Part 11 (Electronic Data).
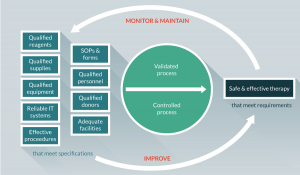
The cGMP regulations for drugs, which include biological drugs, contain minimum requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product. The regulations make sure that a product is safe for use in that it has the ingredients and strength it claims to have. Adherence to the cGMP regulations assures the identity, strength, quality and purity of drug products and is required for manufacturers of medications to adequately control manufacturing operations. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations and maintaining reliable testing of laboratories. The components of a process-oriented quality management system is depicted in Figure 1.
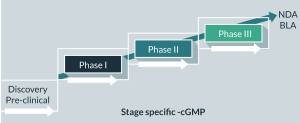
cGMPs should be applied throughout the product lifecycle. GMP stringency increases as the product moves through the product development phases through to commercialization (Figure 2). For instance, the chemistry, manufacturing and control (CMC) information that is included with the early-stage clinical trials may be less detailed than with the licensing application and post-approval amendment applications. Data requirements (such as process validation) for GMP increase as knowledge about the product accumulates. Such information is submitted in the Quality section of the clinical trial and licensing applications. The Quality section contains detailed information on the quality aspects, characteristics and manufacture of the drug substance and drug products. In July 2008, the FDA released a guidance “cGMP for Phase 1 Investigational Drugs” exempting the manufacture of most investigational new drug (IND) and biological products used in Phase 1 clinical trials from complying with 21 CFR part 211 under 21 CFR 210.2(c). Because a Phase 1 clinical trial initially introduces an IND into human subjects, appropriate cGMP helps ensure subject safety. This guidance applies, as part of cGMP, quality control (QC) principles to the manufacture of Phase 1 investigational drugs (i.e., interpreting and implementing cGMP consistent with good scientific methodology), which foster cGMP activities that are more appropriate for Phase 1 clinical trials, improve the quality of Phase 1 investigational drugs, and facilitate the initiation of investigational clinical trials in humans while continuing to protect trial subjects [15].
It is important to note that during product development, the quality and safety of Phase 1 investigational drugs should be maintained, in part, by having appropriate QC procedures in effect. Using established or standardized QC procedures and following appropriate cGMP will also facilitate the manufacture of equivalent or comparable IND product for future clinical trials as needed.
Adherence to cGMP during manufacture of Phase 1 investigational drugs occurs mostly through:
- Well-defined, written procedures
- Adequately controlled equipment and manufacturing environment
- Accurately and consistently recorded data from manufacturing (including testing)
Meeting regulatory requirements for the manufacturing process of CAR-T cells means addressing several technical challenges, which include setting suitable specifications to allow for variability in the starting material (autologous or allogeneic sources). This inherent variability between the sources of patients’ cells is a key reason for the higher batch failure seen with CAR-T cell therapies. Defining specifications for the final product as well as conducting suitably extensive final product characterization will enable comparison of results within trials and following any changes in the product manufacture process or manufacturing site. During the development process, to reach the scale required, further manufacturing optimizations will also be needed to include the use of fully GMP or clinical-grade reagents and ideally remove serum-containing steps. Because the cell therapy field is evolving, GMP quality reagents are not always available and raw materials of biological origin are often required.
Another recognized critical manufacturing step is the transduction of T cells with the viral vector (typically gamma-retrovirus or lentivirus) so as to introduce the genetic modification required to express the CAR. To address the possibility of having a replication competent retrovirus (RCR) develop in retroviral vector-based gene therapy products, there is a requirement for extensive and repeated viral-vector replication-competency testing [16]. RCR may develop at any step during manufacturing, from development of the initial master cell bank through production of the retroviral vector supernatant. In addition, the growth of ex vivo transduced cells provides the potential for amplification of any RCR contaminant, which may be below the level of detection in the retroviral vector supernatant. Therefore, current testing recommendations include testing of material obtained at multiple stages of product manufacture from Master cell banks, end of production cells and vector containing supernatant from the manufacture of the retroviral vector and ex vivo transduced cells that are cultured for more than 4 days after transduction. These recommendations stemmed from early trials but recent experience with more modern vector constructs have shown no evidence of replication competency in vectors designed to be replication incompetent, with no positive replication competency results in samples of retrovirus or lentivirus vector lots that were used for clinical studies in the past 10 years. It would be reasonable to anticipate that the extent of replication-competency testing currently required could be minimized in the future as more clinical safety trials are run and show no evidence of RCR.
Another point of regulatory concern is the current use of gene editing to introduce genetic modifications like the selective deletion of the endogenous T cell receptor in the manufacture of allogeneic CAR-T cells so as to minimize GvHD. With current gene editing technologies, the endogenous TCR could be excised through the use of nucleases such as zinc finger nucleases (ZFNs) [17], transcription activator-like effector nucleases (TALEN) [13,18] and the CRISPR/Cas9 system [19]. The possibility to control and finely tune the targeting specificity of such platforms represents a key issue. These editing tools are not foolproof and may cause gene editing events with the introduction of variable length insertion/deletion (indel) at off-target sites at the site of the break. Off-target sites are defined as sequences within the genome that contain a few mismatches relative to the targeted sequence of interest. The identification of these off-target sites in coding regions (gene, chromosome location, gene function) and non-coding regions and determination that no indels have occurred can be done by using bioinformatics scoring analyses as well as unbiased genome-wide approaches to assess off-target cleavage.
Pre-clinical
In the preclinical area, regulatory guidance is available on the requirements for preclinical testing [20]. To date, the majority of preclinical studies investigating CAR-T cell function have focused upon verifying specificity and potency of anti-tumor activity. This specificity has been generally confirmed by in vitro assays establishing that the T cells endowed with a specific CAR show a significant differential functional response between the target antigen (either as a purified form or present on relevant target cells) and non-specific antigens. Such assays prove CAR function and support translation to the animal model system. For genetically modified T cells, in addition to evaluating the safety of the product and the viral vector involved, the challenge is to evaluate the relevance and utility of relevant efficacy models. In majority of cases, utilization of a risk-based approach will help in the development of the non-clinical strategy.
Within this approach, the following elements should be addressed in pre-clinical studies:
- Scientific basis for conducting clinical trials
- Data to recommend initial safe dose and dose escalation scheme in humans
- Identification of potential target tissue(s) of toxicity/activity
- Identification of parameters for clinical monitoring
- Identification of patient eligibility criteria
- Proof-of-concept studies in relevant animal models
- Extent of functional correction
- Durability of effect
- Determine effective dose level range
- Optimize route of administration/dose selection/dosing regimen
- Collect safety data in the animal models
- Toxicology studies in relevant animal species
- Identify, characterize, quantify the potential local and systemic toxicities
- Identify target organs/sites for toxicity
- Reversibility (acute or chronic toxicities)
- Dose–response relationship
Regulatory authorities generally require information relating to the specific agent, which in this case is the CAR-T cell. It should be expected that the preclinical data that is generated for CAR-T cell products may not be optimally assessed and be as informative as that generated for small molecule pharmaceuticals, particularly since it usually is not feasible to conduct traditional preclinical pharmacokinetic (PK) studies with cell and gene therapy products.
Human T cell engraftment in the majority of immune-compromised mice is limited. Because of the human specificity of the CAR-T cell, studies in standard immunocompetent animal models are not applicable due to the rapid targeting and elimination of human cells by xenogeneic immune reactions and inability of scFv of the CAR-T cell to cross-react with the targeted antigen from other species. Nonetheless, several studies have shown the efficacy of CAR-T cell function using Nude, NOD/SCID or SCID/Beige animals [21–24]. More recently, the availability of the highly immunodeficient murine xenograft model NOG/NSG mouse (NOD/SCID IL-2Rγ-/-) has allowed for both the efficient human T cell engraftment and corresponding recognized human tumor cells. This animal model has been extensively used for the assessment of in vivo activity of CAR-T cell therapies. While these models have the limitations with respect to on-target/off-tumor cytotoxicity and off-target cytotoxicity, and completely eliminate any role of MHC mismatch, they have proven to be extremely useful for assessing in vivo anti-tumor efficacy of CAR-T cells. They have emerged as the de facto standard for assessment of in vivo activity of CAR-T cell products (see articles [25–31] among many published articles). They have proven capable of discriminating the relative anti-tumor activity of different CARs, highlighting their capacity to capture and semi-quantitatively read out useful human in vivo T cell functions (see for example [32]).
However, it has been observed that engrafted T cells can drive a xeno-graft-versus-host disease (xGvHD), which occurs around 50 days after adoptive T cell transfer at doses above 109 cells/kg [33]. The occurrence of xGvHD is associated with transduced cell persistence and this is affected by the cytokine conditions used to culture the cells prior to injection into mice. Consequently, this limits the ability to investigate the long-term effects of CAR-T cells in this model [33–35].
Presently, preclinical model systems are also being used to determine whether the adverse events observed in trials can be recapitulated in mice to permit an examination of underlying mechanisms driving toxicity. At the moment, for the large part, such recapitulation of the clinical situation has not been achieved. Toxicity induced by CD19 CAR-T cells in the BALB/c autologous model system does not completely reflect the toxicity observed in patients, especially considering that IL-6 does not appear to be a major driver of acute toxicity unlike that observed in patients [36]. Moreover, Her2/neu-targeted CAR-T cells have failed to show any evidence of autotoxicity in preclinical models yet the death of a patient receiving such CAR-T cells, potentially as a result of cytokines released due to targeting of Her2/neu present on lung epithelial cells [37] testifies the potency of the therapy and highlights the poor predictive nature of current model systems. The reasons for this dichotomy between preclinical results and the patient situation are not clear but are likely to include factors such as differences in CAR structures, T cell biology between the mouse and human and differential drug metabolic capacity between species [38].
With the observations of toxicity in the clinic, there is now a stronger drive to develop a better understanding of the potential mechanisms of toxicity. Whilst the poor predictive nature of current models may relate to biological differences between rodents and humans, a more robust toxicity testing may be achieved when the CAR is able to engage both human and murine homologs [39].
Good Laboratory Practices (GLPs) are expected to be followed in non-clinical laboratory studies that support or are intended to support applications for research or marketing permits for products regulated by the FDA, including biological products. GLP stipulates a quality system concerned with the organizational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported. GLPs are regulations published in the Code of Federal Regulations (21CFR part 58). The basic elements of GLP are shown in Box 2.
| Box 2: Elements of GLP | |||
|---|---|---|---|
| Personnel | Facility | Documents | Test and Control Articles |
|
|
|
|
Clinical
The clinical trial designs that are initiated for CAR-T cell therapies do not mimic the traditional Phase 1-2-3 development pathway that is usually utilized for the safety and efficacy testing of small molecule pharmaceuticals. Differences in trial design are necessitated by the distinctive features of these products, and may also reflect previous clinical experience. These features, which may contribute to their risks, include the potential for prolonged biological activity after a single administration, a high potential for immunogenicity or the need for relatively invasive procedures to administer the product. Unlike many small molecule pharmaceuticals, the logistics and feasibility of manufacturing a CAR-T cell product more often influence the design of the clinical trials. Therefore, the design of early-phase clinical trials of CAR-T cell products often involves consideration of clinical safety issues, preclinical issues, and CMC issues that are encountered less commonly or not at all in the development of other pharmaceuticals.
Early-phase clinical trials usually start directly in patients and often proceeds in such a manner so as to collect the data required. The IND regulations in 21 CFR Part 312 emphasize the importance of the assessment of trial risks and the safeguards for trial subjects. For early-phase clinical trials, especially first-in-human trials, the primary objective should be an evaluation of safety [40]. Safety evaluation includes an assessment of the nature and frequency of potential adverse reactions and an estimation of the relationship to dose. Considerations are given to optimal dose and administration, defining the appropriate patient population and staggering of the dose escalation. In determining the optimal dose and administration, starting dose level/dose-escalation schemes, the route of administration and dose schedule have to be considered. If immunosuppression is to be used, one has to consider additional factors such as:
- Justification of the dose schedule
- Long-term versus short-term effects of the immunosuppression regimen
- Single drug versus a combination regimen
Besides variations in the investigational product, other differences across CAR-T cell treatment protocols include the type and intensity of lymphodepletion, the timing and dose of anti-CD19 CAR-T cell infusions, and the target patient population and malignancy.
In CAR-modified T cell clinical trials, one factor that has been shown to impact T cell engraftment and proliferation is the use of lymphodepletion chemotherapy in patients prior to T cell infusion [41,42]. This pre-conditioning creates space for the expansion of infused cells, limits the competition for homeostatic gamma chain cytokines IL-2, IL-7 and IL-15, depletes regulatory T cells, and activates the innate immune system. This typically consists of a non-myeloablative chemotherapy regimen usually consisting of a course of cyclophosphamide plus fludarabine prior to infusion. This combination of chemotherapy and pre-conditioning reduces the number of immunosuppressive cells that can inhibit CAR-T cells from being effective. Lymphodepletion may have the additional benefit of tumor cytoreduction, which can potentially improve CAR-T cell treatment efficacy and minimize toxicity. Notably, however, some patients have responded to CAR-T cell therapy in the absence of prior lymphodepletion [43].
For example, in a Phase 1/2a trial, third-generation CD19 CAR-T cell therapy combined with chemotherapy pretreatment resulted in complete response in some lymphoma and leukemia patients, according to data presented by Prof. Angelica Loskog of Uppsala University in Sweden at the CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference, held in September 2015. Loskog and colleagues tested whether combining chemotherapy with pre-conditioning to reduce the number of immunosuppressive cells could improve treatment outcomes. Six patients, three with leukemia and three with lymphoma, had complete remission. Two of them relapsed later. Of the patients who had complete response rates, five had received pre-conditioning therapy. Of the first five patients who did not receive preconditioning treatment the day before CAR-T cell infusion, only one had an initial complete response and the rest had rapid disease progression.
In patients with significant disease burden, especially ALL with extensive marrow infiltration or non-Hodgkin lymphoma with bulky adenopathy, many groups start allopurinol for tumor lysis syndrome (TLS) prophylaxis prior to conditioning chemotherapy or prior to cell infusion [43–45].
It is worth mentioning that in July 2016, Juno Therapeutics reported three deaths from cerebral edema in the so-called ‘ROCKET’ trial that was testing JCAR015, a CAR-T cell against the B cell antigen, CD19, in adult patients with relapsed/refractory ALL. Juno attributed the deaths to the interaction between the preconditioning chemo regimen – consisting of fludarabine and cyclophosphamide – and the CAR-T cells reinfused back into the patient. Since then, 12 new patients were treated with JCAR015 using a pre-conditioning course of cyclophosphamide alone, following which two additional patients died of cerebral edema. Juno executives did not yet have an answer for what factor triggered the new deaths, although Juno’s chief medical officer Mark Gilbert noted the recent cases of cerebral edema followed a similar clinical course as those in July.
Given the extreme potency of CAR-modified T cells, the use of this therapy itself has shown significant toxic potential [38,45–47]. Toxicities range from life threatening cytokine release syndromes (CRS) and macrophage activation syndromes (MAS) to on-target off-tumor toxicity, neurotoxicity and TLS. Guidelines for assessing and managing toxicity following CAR-T cell administration such as the diagnosis and management of CRS has been elucidated by Lee et al. [48]. The hallmark of CRS is immune activation resulting in elevated inflammatory cytokines. Clinical and laboratory measures range from mild CRS (constitutional symptoms and/or grade 2 organ toxicity) to severe CRS (sCRS; grade ≥3 organ toxicity, aggressive clinical intervention, and/or potentially life threatening) [48]. Following diagnosis of CRS, it has been a challenge choosing appropriate therapy to mitigate the physiological symptoms of uncontrolled inflammation without dampening the anti-tumor efficacy of the engineered cells. Systemic corticosteroid administration has been shown to rapidly reverse symptoms of sCRS without compromising initial anti-tumor response [49–50]. However, prolonged use (e.g., >14 days) of high-dose corticosteroids has also resulted in ablation of the adoptively transferred CAR-T cell population potentially limiting their long-term anti-leukemia effect [49]. As an effective alternative, IL-6 receptor (IL-6R) blockade with the FDA-approved mAb, tocilizumab has demonstrated near-immediate reversal of CRS [43,51].
Toxicities caused by CAR-T cells are diverse and not fully understood. Management requires vigilant monitoring, aggressive supportive treatments and, in some cases, intensive care. Administering immunosuppressive agents to decrease toxicity is an evolving practice. Consensus guidelines for grading and managing toxicity will facilitate the administration of CAR-T cells at more centers. Improving the management of CAR-T cell toxicity is one of the most important avenues for overall improvement in the field of CAR-T cell therapies. A review by Brudno and Kochederfer describes the toxicities caused by CAR-T cells and the published approaches used to manage toxicities. It also presents guidelines for treating patients experiencing CRS and other adverse events following CAR-T cell therapy [52]. Since a major objective of early-phase trials is evaluation of safety, early-phase trials should employ general tests and monitoring to look for both expected and unexpected safety issues. General safety monitoring typically includes recording of symptoms and common clinical measurements, such as physical examinations, chemistry profiles, complete blood counts and possibly other examinations that are appropriate for the condition being investigated. An outline of the expected patient and clinical safety monitoring is given in Box 3.
| Box3: Patient and Clinical Safety Monitoring |
|---|
Safety Monitoring plans
Safety Reporting requirements described in21 CFR 312 Pediatric issues |
Trial designs can be challenging along with the requirements for long-term follow-up, usually 15 years, of patients who have received genetically modified therapies. It is most likely that patient registries will be required in order to fulfill long-term follow-up needs and guidance documents related to this are available from the regulators [53–55].
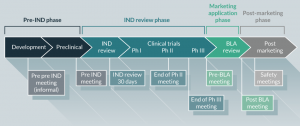
In summary, prior to initiating first-in-human, dose-finding (Phase 1) clinical studies under an IND application, preliminary specifications for product characterization should be in place. These product release specifications for immunotherapeutic products are established based on the IND sponsor’s previous experience with their product (and other similar products, if available) and include analytical procedures based on CFR requirements and FDA-issued guidance documents. As product development proceeds, additional and narrower, specifications for product quality and manufacturing consistency should be implemented based on the data obtained (Figure 3). At the time of initiation of clinical trials intended to support marketing applications (Phase 3), lot-release and other product specifications should be based on all information collected during product development, and consistent with data generated during clinical studies. During the conduct of Phase 3 trials, validation of analytical procedures for product testing should be ongoing or completed.
Financial and competing interests disclosure
The author has no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat. Rev. Immunol. 2009; 9: 704–16.
CrossRef
2. Brentjens R, Hollyman D, Park J et al. Enhanced in-vivo activation of adoptively transferred genetically targeted T cells following cyclophosphamide chemotherapy: initial results from a phase I clinical trial treating CLL patients with autologous CD19 targeted T cells. Blood 2009; 144: Abstract 3436.
3. Till BG, Jensen MC, Wang J et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008; 112: 2261–71.
CrossRef
4. Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015; 125: 4017–23.
CrossRef
5. Jensen MC, Popplewell L, Cooper LJ et al. Anti-transgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010; 16: 1245–56.
CrossRef
6. Savoldo B, Ramos CA, Liu E et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 2011; 121: 1822–6.
https://doi.org/10.1172/JCI46110″ target=”_blank”>CrossRef
7. Maus MV, Grupp SA, Porter DL, June CH. Antibody modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014; 123 (17): 2625–2635.
CrossRef
8. van Schalkwyk MCI, Papa SE, Jeannon JP et al. Design of a Phase I Clinical Trial to Evaluate Intratumoral Delivery of ErbB-Targeted Chimeric Antigen Receptor T-Cells in Locally Advanced or Recurrent Head and Neck Cancer. Hum. Gene Ther. Clin. Dev. 2013; 24(3): 134–42.
CrossRef
9. Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor expressing T cells. Immunol. Rev. 2014; 257(1): 107–26.
CrossRef
10. Qasim W, Zhan H, Samarasinghe S et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017; 9(374): 1–8.
CrossRef
11. Jacoby E, Yang Y, Qin H, Chien CD, Kochenderfer JN, Fry TJ. Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood 2016; 127(10); 1361–70.
CrossRef
12. Dai H, Zhang W, Li X et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. OncoImmunology 2015; 4(11): Article ID e1027469.
CrossRef
13. Poirot L, Philip B, Schiffer-Mannioui C et al. Multiplex genome-edited T-cell manufacturing platform for ‘off-the shelf’ adoptive T-cell immunotherapies. Cancer Res. 2015; 75(18): 3853–64.
CrossRef
14. Thomas RJ, Hourd PC, Williams DJ. Application of process quality engineering techniques to improve the understanding of the in vitro processing of stem cells for therapeutic use. J. Biotechnol. 2008; 136(4): 148–55.
CrossRef
15. FDA Guidance for Industry: CGMP for Phase 1 Investigational Drugs, July, 2008.
16. FDA Guidance for Industry: Supplemental Guidance on Testing for Replication Competent Retrovirus in Retroviral Vector Based Gene Therapy Products and During Follow-up of Patients in Clinical Trials Using Retroviral Vectors, October, 2006.
17. Torikai H, Reik A, Liu PQ et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 2012; 119: 5697–706.
CrossRef
18. Berdien B, Mock U, Atanackovic D, Fehse B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 2014; 21: 539–48.
CrossRef
19. Mandal PK, Ferreira LMR, Collins R et al. Efficient Ablation of Genes in Human Hematopoietic Stem and Effector Cells using CRISPR/Cas9. Cell Stem Cell 2014; 15: 643–52.
CrossRef
20. FDA Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products, November, 2013.
21. Cheadle EJ, Gilham DE, Hawkins RE. The combination of cyclophosphamide and human T cells genetically engineered to target CD19 can eradicate established B-cell lymphoma. Br. J. Haematol. 2008; 142(1): 65–8.
CrossRef
22. Hillerdal V, Ramachandran M, Leja J, Essand M. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer 2014; 14: 30–8.
CrossRef
23. Parente-Pereira AC, Burnet J, Ellison D et al. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J. Clin. Immunol. 2011; 31(4): 710–718.
CrossRef
24. Zhao Y, Moon E, Carpenito C et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010; 70(22): 9053–61.
CrossRef
25. Carpenito C, Milone MC, Hassan R et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. 2009; 106: 3360–65.
CrossRef
26. Gade TPF, Hassen W, Santos E et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005; 65: 9080–88.
CrossRef
27. Gill S, Tasian SK, Ruella M et al. Non-clinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 2014; 123: 2343–54.
CrossRef
28. Hudecek M, Schmitt TM, Baskar S et al. The B-cell tumor associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 2010; 116: 4532–41.
CrossRef
29. Kenderian SS, Ruella M, Shestova O et al. CD33 Specific Chimeric Antigen Receptor T Cells Exhibit Potent Preclinical Activity against Human Acute Myeloid Leukemia. Leukemia 2015; 29(8): 1637–47.
CrossRef
30. Mardiros A, Dos Santos C, McDonald T et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood 2013; 122: 3138–48.
CrossRef
31. Zhou X, Li J, Wang Z et al. Cellular immunotherapy for carcinoma using genetically modified EGFR-specific T lymphocytes. Neoplasia 2013; 15(5): 544–53.
CrossRef
32. Milone MC, Fish JD, Carpenito C et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. J. Am. Soc. Gene Ther. 2009; 17: 1453–64.
CrossRef
33. Alcantar-Orozco EM, Gornall H, Baldan V, Hawkins RE, Gilham DE. Potential limitations of the NSG humanized mouse as a model system to optimize engineered human T cell therapy for cancer. Hum. Gene Ther. Methods 2013; 24(5): 310–20.
CrossRef
34. Hannon M, Lechanteur C, Lucas S et al. Infusion of clinical-grade enriched regulatory T cells delays experimental xenogeneic graft-versus-host disease. Transfusion 2014; 54(2): 353–63.” target=”_blank”>CrossRef
35. Zhou Q, Facciponte J, Jin M, Shen Q, Lin Q. Humanized NOD-SCID IL2rg-/- mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 2014; 344(1): 13–9.
CrossRef
36. Cheadle EJ, Sheard V, Rothwell DG et al. Differential role of Th1 and Th2 cytokines in autotoxicity driven by CD19-specific second-generation chimeric antigen receptor T cells in a mouse model. J. Immunol. 2014; 192(8): 3654–65.
CrossRef
37. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010; 18(4): 843–51.
CrossRef
38. Muruganandan S, Sinal CJ. Mice as clinically relevant models for the study of cytochrome P450-dependent metabolism. Clin. Pharmacol. Ther. 2008; 83(6): 818–28.
CrossRef
39. van der Stegen SJ, Davies DM, Wilkie S et al. Preclinical in vivo modeling of cytokine release syndrome induced by ErbB-retargeted human T cells: identifying a window of therapeutic opportunity? J. Immunol. 2013; 191(9): 4589–98.
CrossRef
40. Code of Federal Regulations Title 21 Part 312 Sec. 21- Phases of an Investigation.
41. Dudley ME, Yang JC, Sherry R et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008; 26(32): 5233–9.
CrossRef
42. Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005; 26(2): 111–7.
CrossRef
43. Maude SL, Frey N, Shaw PA et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014; 371: 1507–17.
CrossRef
44. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993; 90(2): 720–4.
CrossRef
45. Ghorashian S, Pule M, Amrolia P. CD19 chimeric antigen receptor T cell therapy for haematological malignancies. Br. J. Haematol. 2015; 169(4): 463–78.
CrossRef
46. Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: Case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 2010; 18(4): 666–68.
CrossRef
47. Kochenderfer JN, Dudley ME, Feldman SA et al. B-cell depletion and remissions of malignancy along with cytokine associated toxicity in a clinical trial of anti-CD19 chimeric antigen-receptor-transduced T cells. Blood 2012; 119(12): 2709–20.
CrossRef
48. Lee DW, Gardner R, Porter DL et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124: 188–95.
CrossRef
49. Davila ML, Riviere I, Wang X et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014; 6: 224–25.
CrossRef
50. Lee DW, Kochenderfer JN, Stetler-Stevenson M et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–28.
CrossRef
51. Grupp SA, Kalos M, Barrettm D et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013; 368: 1509–.
CrossRef
52. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016; 127: 3321–30.
CrossRef
53. FDA Guidance for Industry: Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products, June 2015.
54. FDA Guidance for Industry: E6 Good Clinical Practice: Consolidated Guidance, April 1996.
55. FDA Guidance for Industry: Gene Therapy Clinical Trials- Observing Subjects for Delayed Adverse Events, November 2006.
56. FDA Cellular & Gene Therapy Guidances. Website
57. ICH Quality Guidelines. Website
Affiliations
Shirley M Bartido
Director of Regulatory Affairs
Cellectis Inc. NY, USA
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.