Daidzein analog 2l enhances osteogenic differentiation of BMSCs and ASCs seeded on PLGA scaffolds and heals critical size calvarial defects
Cell Gene Therapy Insights 2017; 3(3), 197-210.
10.18609/cgti.2017.032
Repair of craniomaxillofacial defects continues to present a significant challenge due to the limited amount of autogenous and allogeneic bone for reconstruction. Significant advances in the development of biodegradable polymeric material and the understanding of adult stem cells will yield new tissue engineering approaches to reduce the burden of craniomaxillofacial defects. Tissue engineering approaches have utilized biodegradable scaffolds in combination with bone marrow-derived mesenchymal stem cells (BMSCs) or adipose-derived stromal/stem cells (ASCs) and growth factors to augment the healing process. While this approach is promising, identification of additional factors that play a role in bone healing may further accelerate the bone regeneration process. The osteoinductive properties of daidzein analogs in monolayer adherent cultures have previously been demonstrated. In the current study, BMSCs and ASCs were seeded on poly lactic-co-glycolic acid scaffolds and treated with daidzein analogs in vitro. Scaffolds seeded with BMSCs and ASCs and treated with daidzein analogs demonstrated enhanced osteogenic differentiation in vitro. Additional in vivo analysis of the daidzein analog was conducted in the critical size calvarial defect model. Calvarial defects treated with scaffolds seeded with BMSCs or ASCs and exposed to the daidzein analog 2l also demonstrated enhanced osteogenesis. Histological analysis of the liver and kidney demonstrated no pathological findings. Collectively, these results suggest that daidzein analogs utilized in combination with biodegradable scaffolds and adult stem cells may be a safe and efficacious combination to promote the regeneration of bone and thus reduce the burden of craniomaxillofacial defects.
Craniomaxillofacial defects secondary to trauma, tumor resection or congenital malformations remain a challenge to treat due to inherent limitations, such as donor-site morbidity, graft resorption and infection [1]. Traditionally, reconstructive options included autogenous grafts; however, autogenous bone grafts derived from the patient are limited in availability and create a secondary deformity. Furthermore, in the osteoporotic patient, these bone grafts are likely to be osteoporotic as well, limiting their ability to heal fracture sites. Significant strides have recently been made in the field by combining osteoinductive scaffolds with cellular components and growth factors in a tissue engineering approach [2,3]. Recently, studies have utilized tissue engineering approaches to reduce the burden of craniomaxillofacial defects [4,5]. During the past two decades, significant advances have been made in the development of biodegradable polymeric material for bone tissue engineering. Degradable polymeric biomaterials are preferred candidates for the development of 3D porous structures such as scaffolds for tissue engineering [6,7]. Several of the biodegradable constructs have been FDA approved in recent years, resulting in the increased use of these scaffolds both for research purposes and in the clinical setting.
Several studies have demonstrated the osteoinductive effects of adult stem cells seeded on scaffolds and the potential of this method to enhance bone regeneration or repair. Adult stem cells are the preferred first choice in bone tissue engineering due to their ability to self-renew and their capacity to undergo osteogenic differentiation. While bone marrow-derived mesenchymal stem cells (BMSCs) were the first to be characterized, adipose-derived stromal/stem cells (ASCs) have gained significant attention due to the ease of isolation, larger quantities of cells obtained per weight of tissue and similar differentiation capacity to their BMSC counterparts [8–10]. Nevertheless, studies investigating the therapeutic efficacy of BMSCs and ASCs have shown that both cell types are effective against a wide range of diseases and have regenerative properties [11]. While the capacity to differentiate into adipogenic, osteogenic and chondrogenic lineage cells explains part of their regenerative potential, studies have also shown that these cells secrete an abundance of cytokines and chemokines that mediate the inflammatory process and assist in revascularization of the tissue [12,13].
Recently, the addition of growth factors to osteoinductive scaffolds containing adult stem cells have been shown to provide a microenvironment that enhances the growth and differentiation of cells. Cytokines and osteoinductive growth factors play a major role in the recruitment of osteogenic progenitors to the site of bone formation. These factors further promote the differentiation of these cells into osteogenic lineage cells. Of these molecules, bone morphogenetic proteins (BMPs) have been the most extensively studied, as they are potent stimulators of osteogenesis. Studies have shown that BMPs induce the mitogenesis of BMSCs, ASCs and other osteoprogenitors, and enhance their differentiation into osteoblasts [14–17]. Recent clinical studies have also attempted to augment the osteogenic microenvironment of scaffolds through the incorporation of recombinant BMP ligands. However, the results from these studies have been inconclusive due to the fact that BMP ligands can have a catabolic effect on bone through expression of the receptor activator of nuclear factor kappa B (RANK) ligand–osteoprotegerin pathway. Therefore, the development of alternative compounds that can be incorporated into a bone tissue engineering approach is necessary.
Rather than the recombinant protein-based strategies, we have begun to focus on plant-derived hormones or phytoestrogens due to their potential beneficial role in preventing and treating osteoporosis [18,19]. Recently, phytoestrogens have gained significant attention due to their structural similarity to estradiol and capacity to bind to estrogen receptors. The most commonly studied phytoestrogens include genistein, daidzein and glycitein, and the mechanism of action of these compounds has been shown to involve RUNX2, through binding and/or phosphorylation of these genes, resulting in the commitment of osteoprogenitor cells to the osteoblastic lineage [20]. While these compounds have osteoinductive effects on BMSCs and ASCs, previous studies have synthesized analogs to these compounds to further increase their osteogenic effects [21–23]. Stem cells and osteoprogenitor cells treated with these daidzein analogs demonstrated enhanced osteogenic differentiation and upregulation of osteogenic genes in monolayer culture [21–23]. These daidzein analogs may also improve osteogenic differentiation of BMSCs and ASCs seeded on scaffolds.
Therefore, in the current study, a bone tissue engineering approach was utilized, whereby critical size calvarial defects were treated with BMSC- or ASC-seeded poly lactic-co-glycolic acid (PLGA) scaffolds that were treated with daidzein analogs. We previously designed daidzein analog 2g and 2l, and demonstrated the effects of these analogs on enhancing osteogenic differentiation [22]. Daidzein analogs 2g and 2l have a modification of the 7-OH with a cyclopentyl and an allyl, respectively [22]. Both compounds were found to enhance osteogenic differentiation and enhance the transcription of key osteogenic genes [23]. In the current study, the exposure to the daidzein analog 2l improved bone formation in the calvarial defect, regardless of the cell type. To determine the safety of the daidzein analog 2l, organs were assessed for malignancy. No abnormal pathological findings were observed in mice receiving daidzein analogs. Collectively, this study illustrates the safety and efficacy of daidzein analogs to enhance osteogenic differentiation in a calvarial defect model.
Materials & methods
Chemicals
Type 1 collagenase, bovine serum albumin (BSA, fraction V), calcium chloride, dexamethasone, isobuytlmethylxanthine, indomethacin, ascorbate 2-phosphate, β-glycerol phosphate, Alizarin Red S, cetylpyridinium chloride (CPC), estradiol, daidzein, poly lactic-co-glycolic acid (PLGA), Aniline Blue, and Permount mounting medium were purchased from Sigma-Aldrich (St Louis, MO, USA). Hematoxylin and Eosin were purchased from Richard-Allan Scientific (Thermo Fisher Scientific; Waltham, MA, USA) and Movat’s Pentachrome solutions were purchased from Electron Microscopy Sciences (Hatfield, PA, USA). Daidzein analogs (i.e., 2g and 2l) were synthesized in our lab as described previously [24,25].
Human subjects
Primary human BMSCs were obtained from six healthy consenting Caucasian female donors under a protocol approved by the Tulane University Institutional Review Board. The cells were prepared from bone marrow aspirates taken from the iliac crest of six individuals. Nucleated cells were isolated using Ficoll-Paque density gradient (Amersham Pharmacia Biotech; Milwaukee, WI, USA) and resuspended in complete culture media (CCM), which consisted of α-Minimal Essential Medium (Gibco; Grand Island, NY, USA), 20% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA), 100 units per ml penicillin/100 µg/mL streptomycin (P/S; Gibco), and 2 mM L-glutamine (Gibco). The cells were then seeded on a 150-cm2 culture dish (Nunc; Rochester, NY, USA) and maintained in a humidified 5% CO2 incubator at 37°C. Medium was changed every 3-4 days. When the cultures reached 70% confluence, the cells were harvested with 0.25% trypsin/1mM EDTA (Gibco) and cryopreserved prior to experimental use.
Primary human ASCs were obtained from six healthy consenting Caucasian female donors undergoing elective liposuction procedures under a protocol approved by the Pennington Biomedical Research Center Institutional Review Board. ASCs were isolated from processed lipoaspirates from the subcutaneous adipose tissue of subjects. Lipoaspirates were purified in 0.1% type I collagenase and 1% bovine serum albumin (BSA) dissolved in 100ml of phosphate buffered saline (PBS, Invitrogen, Grand Island, NY, USA) supplemented with 2 mM calcium chloride. The mixture was placed in a 37oC shaking water bath at 75 rpm for 60 min and then centrifuged to remove oil, fat, primary adipocytes and collagenase solution, leaving behind a pellet of cells. Cells were resuspended in CCM, plated on 150 cm2 culture dishes, and maintained in a humidified 5% CO2 incubator. Fresh medium was added every 2–3 days until cells achieved 80–90% confluence and were harvested with 0.25% trypsin/1mM EDTA and cryopreserved prior to experimental use. All donors were characterized by our group for differentiation potential, self-renewal capacity, cell surface antigen expression and morphological appearance, as previously described [23].
PLGA scaffold preparation
PLGA scaffolds were fabricated from 85/15 PLGA by solvent casting and lyophilization process, as previously described [26]. The mixture was poured into a polydimethylsiloxane mold (Dow Corning; Midland, MI, USA) to generate 4-mm diameter scaffolds and immediately transferred to a -20oC freezer. Once the solution solidified, the samples were immediately freeze-dried for 4 hours and sterilized in 70% ethanol prior to experimentation.
Cell culture
Frozen vials of BMSCs and ASCs were separately thawed and cultured on 150 cm2 culture dishes (Nunc, Rochester, NY, USA) in 25 ml CCM and incubated at 37°C with 5% humidified CO2. After 24 hours, viable cells were harvested with 0.25% trypsin/1mM EDTA and replated at 100 cells/cm2 in CCM. Medium was changed every 2–3 days. For all experiments, sub-confluent cells (≤70% confluent) between passages 2–6 were used. Where indicated, CCM was made with charcoal dextran stripped FBS (CDS-CCM; Atlanta Biologicals, Lawrenceville, GA) in order to deplete the medium of growth factors and hormones.
Cell seeding on PLGA scaffolds
All scaffolds were soaked in PBS for 1 hour prior to cell seeding to remove residual ethanol during the sterilization process. Equal number of BMSCs from all BMSC donors (n = 6) or equal number of ASCs from all ASC donors (n = 6) were pooled and directly loaded onto a single face of each 4 mm PLGA scaffold at a concentration of 6,000 cells per μl for a total volume of 25 μl for 30 minutes. The scaffolds were subsequently submerged in 100 μl of growth medium overnight at 37°C with 5% humidified CO2.
Osteogenic differentiation & staining of PLGA scaffolds
To assess osteogenic differentiation of cell seeded scaffolds, PLGA scaffolds were cultured for 7 or 14 days in CDS-CCM or osteogenic differentiation medium (CDS-ODM), which consisted of CDS-CCM supplemented with 50 µM ascorbate 2-phosphate, 10 mM β-glycerol phosphate and 10 nM dexamethasone. Where indicated, medium was supplemented with vehicle (PBS), estradiol (10 nM), 2g (1 μM) or 2l (1 μM) and changed every 2–3 days (Table 1). After 7 or 14 days, scaffolds were rinsed with PBS, fixed in 70% ethanol for 30 minutes at room temperature and stained with 40 mM Alizarin Red (pH 4.1) for 1 hour to visualize calcium deposition in the cell seeded scaffolds. For quantification, Alizarin Red was eluted from each scaffold with 10% CPC and read at 584 nm (FLUOstar optima; BMG Labtech, Cary, NC, USA).
Critical size calvarial defect model
All procedures involving animals were conducted in compliance with State and Federal law, standards of the US Department of Health and Human Services and guidelines established by the Tulane University Institutional Animal Care and Use Committee (IACUC). All protocols were approved by the Tulane University IACUC. Male Crl:NU-Foxn1nu CD-1 nude mice (60 days old) were obtained from Harlan Laboratories (Indianapolis, IN, USA). Briefly, critical size (4 mm) calvarial defects were created in the right parietal bone of adult male mice. After anesthesia, the surgical site was cleaned with povidone/iodine solution (Ricca Chemical; Thermo Fisher Scientific), and an incision was made just off the sagittal midline. A unilateral 4 mm full thickness defect was created using diamond-coated trephine bits in the nonsuture-associated portion of the right parietal bone, taking extreme care to avoid dural injury.
Mice were divided into ten treatment groups (n = 3 per group): no scaffold (empty); scaffold alone; scaffold and estradiol; scaffold and 2l; BMSC-seeded scaffold; BMSC-seeded scaffold and estradiol; BMSC-seeded scaffold and 2l; ASC-seeded scaffold; ASC-seeded scaffold and estradiol; or ASC-seeded scaffold and 2l (Table 1). In preparation for implantation, scaffolds were pre-treated with estradiol or 2l on the day of cell seeding. The rationale for pre-treatment is to reduce the number of post-operative treatments, thereby increasing the ease of clinical translation of this therapy. Following implantation, the skin was sutured and animals were monitored per established post-operative protocols. Mice in the treatment groups were delivered 10 nM of estradiol or 1uM of 2l in a final volume of 10 µl resuspended in PBS on post-operative days 3 and 7. The skin overlying the scaffold was minimally displaced superiorly to allow for the subcutaneous delivery of the compounds directly over the scaffold through a 25-mm gauge needle. Care was taken not to puncture the scaffold and the underlying dura and brain matter. After 8 weeks, animals were euthanized by cervical dislocation after exposure to CO2. Organs were removed and fixed in 10% neutral buffered formalin for additional analyses.
| Table 1 Experimental Groups | |||
|---|---|---|---|
| Treatment groups | Scaffold | Cells | Treatment |
| In vitro | |||
| Group 1 | PLGA | BMSCs | – |
| Group 2 | PLGA | ASCs | – |
| Group 3 | PLGA | BMSCs | Estradiol |
| Group 4 | PLGA | ASCs | Estradiol |
| Group 5 | PLGA | BMSCs | Daidzein |
| Group 6 | PLGA | ASCs | Daidzein |
| Group 7 | PLGA | BMSCs | 2g |
| Group 8 | PLGA | ASCs | 2g |
| Group 9 | PLGA | BMSCs | 2l |
| Group 10 | PLGA | ASCs | 2l |
| In vivo | |||
| Group 1 | – | – | – |
| Group 2 | PLGA | – | – |
| Group 3 | PLGA | BMSCs | – |
| Group 4 | PLGA | ASCs | – |
| Group 5 | PLGA | – | Estradiol |
| Group 6 | PLGA | BMSCs | Estradiol |
| Group 7 | PLGA | ASCs | Estradiol |
| Group 8 | PLGA | – | 2l |
| Group 9 | PLGA | BMSCs | 2l |
| Group 10 | PLGA | ASCs | 2l |
| PLGA: Polylactic-co-glycolic acid; 2g: Daidzein analog 2g; 2l: Daidzein analog 2l. | |||
Micro-computed tomography
Micro-computed tomography (microCT) was performed ex vivo 8 and 16 weeks after surgery. The craniums were dissected free from soft tissue and fixed in 10% NBF. The dorsal half of the cranium was scanned in a dorsal plane (Scanco model 40; Scanco Medical AG, Basserdorf, Switzerland) in fluid (70% ethanol) at 55kV, 0.3-second integration time, with a 15-µm3 voxel size and 15-µm slice thickness. The region of interest was defined as a 4-mm diameter core including the defect from dorsal to ventral. Bone Hounsfield units were standardized based on a phantom. Bone volume relative to total volume for each group was analyzed and normalized to the control group.
Histological analysis
Calvaria were harvested, formalin fixed, decalcified in 10% EDTA (Sigma-Aldrich), paraffin embedded and sectioned at 5-µm thickness. Kidney and liver sections were harvested, fixed in formalin, paraffin embedded and sectioned at 5-µm thickness. Tissue sections were deparaffinized, rehydrated in Sub-X and graded solutions of ethanol. The kidney and liver sections were stained with Hematoxylin and Eosin, while calvarial sections were stained with Aniline Blue. After staining, slides were dehydrated in graded solutions of ethanol and Sub-X in the final step, and sealed with Permount Mounting Medium. Images were acquired with the ScanScope CS2 (Aperio, Vista, CA) running Image Scope (Aperio).
Statistical analysis
All values are presented as means ± standard error of the means as indicated in the figure legends. The statistical differences between three or more groups were determined by a one-way analysis of variance (ANOVA), followed by post-hoc Tukey multiple comparison tests. Statistical significant was set at p < 0.05. Analysis was performed using Prism (Graphpad Software, San Diego, CA, USA).
Results
Daidzein analogs & estradiol enhance osteogenic differentiation of seeded PLGA scaffolds
BMSCs and ASCs were seeded within PLGA scaffolds and exposed to CDS-ODM supplemented with vehicle, estradiol, daidzein, 2g or 2l. After 1 week, BMSCs exposed to estradiol and 2l demonstrated enhanced osteogenesis to 4.9-fold and 2.0-fold, respectively, compared to vehicle-treated BMSCs (Figure 1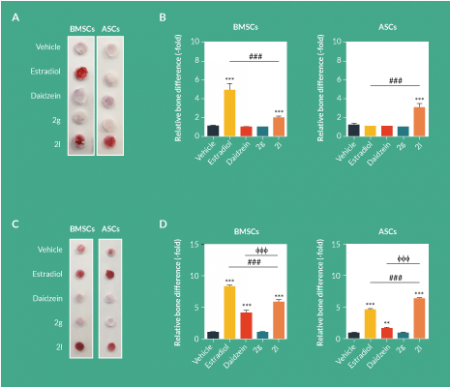
After 2 weeks, both BMSCs and ASCs treated with estradiol demonstrated robust induction of osteogenic differentiation by 8.9-fold and 4.9-fold, respectively (Figure 1C–D). 2l induced osteogenic differentiation in BMSCs (6.0-fold) and ASCs (6.6-fold; Figure 1C–D). Although daidzein induced osteogenesis, the levels of induction in BMSCs and ASCs were significantly lower than those observed in estradiol and 2l (Figure 1C–D). 2g had no osteogenic effects on BMSCs or ASCs seeded on scaffolds (Figure 1C–D).
Daidzein analog 2l demonstrates enhanced osteogenic potential in vivo
To assess the effects of daidzein analogs in vivo, scaffolds were left unseeded or seeded with BMSCs and ASCs and pretreated with one dose of vehicle, estradiol or 2l. Critical size calvarial defects were generated and seeded scaffolds were applied. Due to the poor osteogenic response displayed with daidzein and 2g, additional in vivo experiments only examined the therapeutic efficacy of estradiol and 2l. After 8 weeks, the addition of estradiol and 2l to empty scaffolds enhanced osteogenesis by 5.2-fold and 3.5-fold, respectively, compared to mice treated with vehicle (2.0-fold; ). The addition of BMSCs to scaffolds enhanced osteogenesis in calvarial defects by 5.5-fold, and calvarial defects treated with BMSC-seeded scaffolds and both estradiol and 2l enhanced osteogenesis to 10.6-fold and 15.1-fold, respectively (Figure 2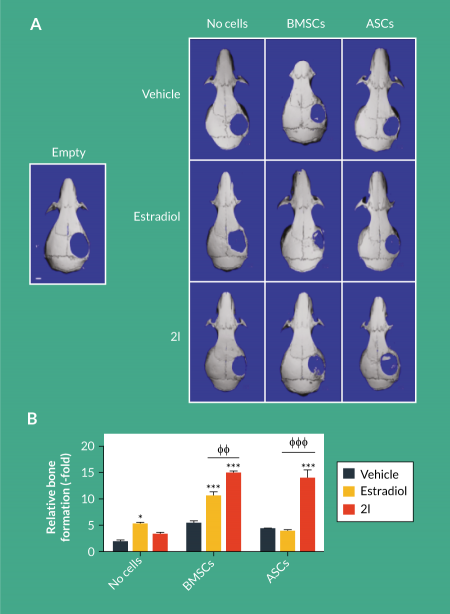
Histological analysis of the calvarial defects by aniline blue staining demonstrated enhanced collagen deposition following treatment with cells and estradiol or 2l (Figure 3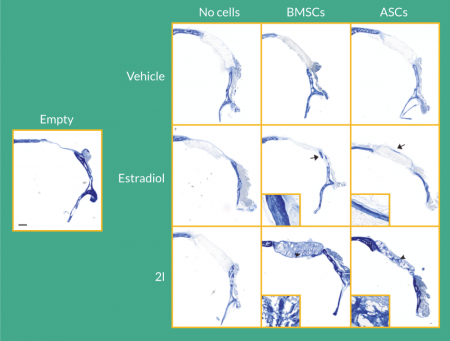
Daidzein analog 2l does not induce cytotoxicity in secondary organs
While it is unlikely that daidzein analog 2l treatment would be toxic to secondary organs, histological analysis of the kidney and liver was performed 8 weeks after delivery of the compounds. Delivery of 2l to BMSC-seeded scaffolds or ASC-seeded scaffolds to calvarial defects did not result in pathological findings in the kidney and liver (Figure 4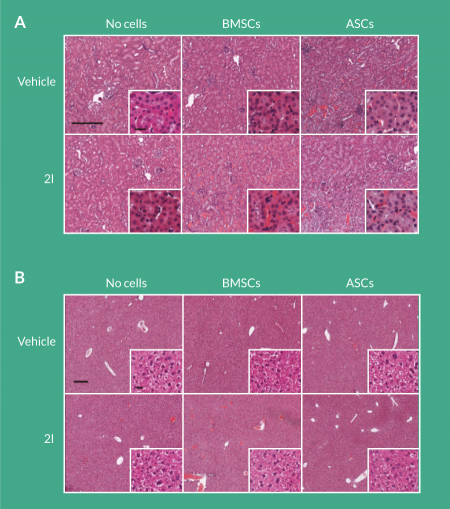
Discussion
BMSCs and ASCs were seeded on PLGA scaffolds and treated with estradiol or daidzein analog 2l. Compared to the respective vehicle-treated groups, estradiol and daidzein enhanced the osteogenic differentiation of BMSCs and ASCs on PLGA scaffolds in vitro. Furthermore, in vivo animal experiments confirmed the therapeutic efficacy of the daidzein analog 2l to enhance the therapeutic efficacy of stem cell- and scaffold-based tissue engineering approaches. These results are consistent with Liu et al. who demonstrated enhanced osteogenic differentiation of pre-osteoblasts seeded in hyaluronic acid-modified chitosan/collagen/nano-hydroxyapatite composite scaffolds and treated with the phytoestrogen α-zearalanol [27]. Osteogenic phenotype was increased and maintained with enhanced collagen type Ia and enhanced alkaline phosphatase activity [27]. Similarly, Wang and colleagues demonstrated that treatment with the phytoestrogen icaritin promoted new bone formation within the bone defect and neovascularization of the surrounding tissue [28]. Collectively, these studies suggest that while scaffold-based tissue engineering approaches supplemented with stem cells may enhance osteogenesis [29,30], the addition of phytoestrogens may further promote new bone formation.
In the present study, we observed differential responses of BMSCs and ASCs to estradiol, while the response to the daidzein analog 2l was consistent in both BMSCs and ASCs in vivo. Estradiol promoted new bone formation in calvarial defects treated with BMSC-seeded scaffolds, whereas estradiol did not enhance osteogenic differentiation within the calvarial defects implanted with ASC-seeded scaffolds. In contrast to the in vivo findings, the in vitro findings demonstrated similar trends between BMSC- and ASC-seeded scaffolds. Previous studies have shown that BMSCs and ASCs treated with estradiol responded similarly to the external stimuli [31,32]. Thus, the difference in response is likely associated with other cell types found within the microenvironment of the calvarial defects treated with BMSC-seeded scaffolds, compared to ASC-seeded scaffolds. In contrast, the daidzein analog 2l promoted osteogenesis of BMSCs and ASCs seeded on scaffolds both in vitro and in vivo. These results suggest that the daidzein analog may be a more robust stimulator of osteogenesis.
Interestingly, daidzein analog 2l demonstrated enhanced osteogenic differentiation compared to estradiol in vivo, whereas estradiol demonstrated enhanced osteogenic differentiation compared daidzein analog 2l in vitro. The interplay between additional cell types in vivo may explain the differences observed between the in vitro and in vivo effects of estradiol and daidzein. It is likely that daidzein analog 2l is interacting with immune cells, osteoblast or osteoclast to enhance osteogenesis and reduce osteoclastogenesis. Studies performed by Rassi et al. and Ohtomo et al. demonstrated a significant inhibition of osteoclast formation induced by 1α,25-dihydroxyvitamin D3 following treatment with daidzein in a dose-dependent manner, supporting the potential influence of daidzein and potentially daidzein analogs on other cellular lineages [33,34].
Previous studies have also shown that PLGA scaffolds seeded with BMSCs and ASCs produce significant intramembranous bone formation within 2 weeks, and complete closure have been observed by 8 weeks [35,36]. It should be noted that the scaffolds used in these other studies were coated with hydroxyapatite, which has been shown to enhance osteogenic differentiation of stem cells and osteoprogenitor cells [37–39]. In the current study, hydroxyapatite was not included in the makeup of the scaffolds in order to assess the direct efficacy of our novel therapeutics. Additionally, hydroxyapatite can cause uncontrolled, prolonged inflammation, which may limit its clinical use [40]. Future studies investigating the osteoinductive effects of daidzein analog 2l treatment of BMSC-seeded or ASC-seeded scaffolds composed of PLGA and hydroxyapatite will determine whether daidzein analog 2l can work synergistically with the scaffold and cells to promote new bone formation. Additional studies utilizing nanoparticles to load daidzein analog 2l onto the scaffold may prove to be an extended, slow release mechanism to enhance osteogenesis. Nevertheless, the current study provides insight into the use of phytoestrogens to enhance osteogenesis of BMSC- or ASC-seeded scaffolds for bone tissue engineering approaches.
Due to the structural similarity between estradiol and daidzein analog 2l and the increased incidence of breast and endometrial cancer associated with hormone replacement therapy with estradiol, the safety of daidzein analog 2l is of particular interest. With regards to the safety of phytoestrogens, the secondary organs were examined with histology. Consistent with previously published studies, no abnormalities were observed at the incision site or within the organs of mice treated with daidzein analog 2l [41]. Previous studies investigating the safety of phytoestrogens have shown these compounds to have antioxidative and anti-angiogenic properties [42–45]. Additional studies have shown that phytoestrogens act on neoplastic cells by inhibiting genes associated with cell cycle progression and enhancing genes involved in programmed cell death [46,47]. These results suggest that phytoestrogens do not have the cancer promoting effects of estrogens. However, additional long-term animal studies and clinical trials are necessary to determine the safety of the daidzein analog 2l.
Collectively, these results suggest that this daidzein analog may be a suitable molecule to enhance osteogenic differentiation of BMSC- or ASC-seeded scaffolds. The daidzein analog 2l, used in combination with other bone stimulating factors, may lead to the development of healthy and physiologically normal bone and thus aid in reducing the burden of craniomaxillofacial defects.
Financial & competing interests disclosure
The first and corresponding authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. J Gimble is the co-owner and Chief Scientific Officer of LaCell LLC. No writing assistance was utilized in the production of this manuscript.
References
1. Warren SM, Fong KD, Chen CM et al. Tools and techniques for craniofacial tissue engineering. Tissue Eng. 2003; 9: 187–200.
CrossRef
2. Sandor GK. Tissue engineering of bone: Clinical observations with adipose-derived stem cells, resorbable scaffolds, and growth factors. Ann. Maxillofac. Surg. 2012; 2: 8–11.
CrossRef
3. Sandor GK, Numminen J, Wolff J et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl. Med. 2014; 3: 530–40.
CrossRef
4. Ulery BD, Nair LS, Laurencin CT. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011; 49: 832–64.
CrossRef
5. Nair LS, Laurencin CT. Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv. Biochem. Eng. Biotechnol. 2006; 102: 47–90.
CrossRef
6. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005; 26: 5474–91.
CrossRef
7. Tevlin R, McArdle A, Atashroo D et al. Biomaterials for craniofacial bone engineering. J. Dent. Res. 2014; 93: 1187–95.
CrossRef
8. Zuk PA, Zhu M, Mizuno H et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7: 211–28.
CrossRef
9. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987; 20: 263–72.
CrossRef
10. Asatrian G, Pham D, Hardy WR, James AW, Peault B. Stem cell technology for bone regeneration: current status and potential applications. Stem Cells Cloning 2015; 8: 39–48.
11. Liao HT, Chen CT. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014; 6: 288–95.
CrossRef
12. Jones E, Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury 2011; 42: 562–8.
CrossRef
13. Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy 2005; 7: 36–45.
CrossRef
14. Chen L, Lu X, Li S et al. Sustained delivery of BMP-2 and platelet-rich plasma-released growth factors contributes to osteogenesis of human adipose-derived stem cells. Orthopedics 2012; 35: e1402–9.
CrossRef
15. Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007; 28: 2087–96.
CrossRef
16. Oest ME, Dupont KM, Kong HJ, Mooney DJ, Guldberg RE. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J. Orthop. Res. 2007; 25: 941–50.
CrossRef
17. Lee JY, Zhou Z, Taub PJ et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PloS One 2011; 6: e17531.
CrossRef
18. Duncan AM, Phipps WR, Kurzer MS. Phyto-oestrogens. Best Pract. Res. Clin. Endocrinol. Metab. 2003; 17: 253–71.
CrossRef
19. Schilling T, Ebert R, Raaijmakers N, Schutze N, Jakob F. Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. J. Steroid Biochem. Mol. Biol. 2014; 139: 252–61.
CrossRef
20. Bhukhai K, Suksen K, Bhummaphan N et al. A phytoestrogen diarylheptanoid mediates estrogen receptor/Akt/glycogen synthase kinase 3beta protein-dependent activation of the Wnt/beta-catenin signaling pathway. J. Biol. Chem. 2012; 287: 36168–78.
CrossRef
21. Yadav DK, Gautam AK, Kureel J et al. Synthetic analogs of daidzein, having more potent osteoblast stimulating effect. Bioorg. Med. Chem. Lett. 2011; 21: 677–81.
CrossRef
22. Strong AL, Jiang Q, Zhang Q et al. Design, synthesis, and osteogenic activity of daidzein analogs on human mesenchymal stem cells. ACS Med. Chem. Lett. 2014; 5: 143–8.
CrossRef
23. Strong AL, Ohlstein JF, Jiang Q et al. Novel daidzein analogs enhance osteogenic activity of bone marrow-derived mesenchymal stem cells and adipose-derived stromal/stem cells through estrogen receptor dependent and independent mechanisms. Stem Cell Res. Ther. 2014; 5: 105.
CrossRef
24. Strong AL, Jiang Q, Zhang Q et al. Design, synthesis, and osteogenic activity of daidzein analogs on human mesenchymal stem cells. ACS Med. Chem. Lett. 2013 [Under Review].
25. Jiang Q, Payton-Stewart F, Elliott S et al. Effects of 7-O Substitutions on Estrogenic and Anti-Estrogenic Activities of Daidzein Analogues in MCF-7 Breast Cancer Cells. J. Med. Chem. 2010; 53: 6153–63.
CrossRef
26. Qureshi AT, Doyle A, Chen C et al. Photoactivated miR-148b-nanoparticle conjugates improve closure of critical size mouse calvarial defects. Acta Biomater. 2015; 12: 166–73.
CrossRef
27. Liu L, Guo Y, Chen X et al. Three-dimensional dynamic culture of pre-osteoblasts seeded in HA-CS/Col/nHAP composite scaffolds and treated with alpha-ZAL. Acta Biochim. Biophys. Sin. 2012; 44: 669–77.
CrossRef
28. Wang XL, Xie XH, Zhang G et al. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J. Orthop. Res. 2013; 31: 164–72.
CrossRef
29. Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 2009; 20: 646–55.
CrossRef
30. Arvidson K, Abdallah BM, Applegate LA et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011; 15: 718–46.
CrossRef
31. Hong L, Colpan A, Peptan IA, Daw J, George A, Evans CA. 17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007; 13: 1197–203.
CrossRef
32. Hong L, Colpan A, Peptan IA. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006; 12: 2747–53.
CrossRef
33. McCollough EG, Fedok FG. The lateral crural turnover graft: correction of the concave lateral crus. Laryngoscope 1993; 103: 463–9.
CrossRef
34. Rassi CM, Lieberheer M, Chaumaz G, Pointillart A, Cournot G. Down-regulation of osteoclast differentiation by daidzein via caspase 3. J. Bone Miner. Res. 2002; 17: 630–8.
CrossRef
35. Levi B, James AW, Nelson ER et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PloS One 2010; 5: e11177.
CrossRef
36. Cowan CM, Shi YY, Aalami OO et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004; 22: 560–7.
CrossRef
37. Liu H, Xu GW, Wang YF et al. Composite scaffolds of nano-hydroxyapatite and silk fibroin enhance mesenchymal stem cell-based bone regeneration via the interleukin 1 alpha autocrine/paracrine signaling loop. Biomaterials 2015; 49: 103–12.
CrossRef
38. Chang YL, Stanford CM, Keller JC. Calcium and phosphate supplementation promotes bone cell mineralization: implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000; 52: 270–8.
<a href=”https://doi.org/10.1002/1097-4636(200011)52:23.0.CO;2-1″ target=”_blank”>CrossRef
39. Budiraharjo R, Neoh KG, Kang ET. Hydroxyapatite-coated carboxymethyl chitosan scaffolds for promoting osteoblast and stem cell differentiation. J. Colloid Interface Sci. 2012; 366: 224–32.
CrossRef
40. Velard F, Laurent-Maquin D, Guillaume C et al. Polymorphonuclear neutrophil response to hydroxyapatite particles, implication in acute inflammatory reaction. Acta Biomater. 2009; 5: 1708–15.
CrossRef
41. Munro IC, Harwood M, Hlywka JJ et al. Soy isoflavones: a safety review. Nutr. Rev. 2003; 61: 1–33.
CrossRef
42. Li Y, Chen H, Hardy TM, Tollefsbol TO. Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PloS One 2013; 8: e54369.
CrossRef
43. Piao M, Mori D, Satoh T, Sugita Y, Tokunaga O. Inhibition of endothelial cell proliferation, in vitro angiogenesis, and the down-regulation of cell adhesion-related genes by genistein. Combined with a cDNA microarray analysis. Endothelium 2006; 13: 249–66.
CrossRef
44. Ullah MF, Ahmad A, Zubair H et al. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011; 55: 553–9.
CrossRef
45. Choi EJ, Kim GH. Antiproliferative activity of daidzein and genistein may be related to ERalpha/c-erbB-2 expression in human breast cancer cells. Mol. Med. Rep. 2013; 7: 781–4.
46. Moreira AC, Silva AM, Santos MS, Sardao VA. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J. Steroid Biochem. Mol. Biol. 2014; 143: 61–71.
CrossRef
47. Wietrzyk J, Grynkiewicz G, Opolski A. Phytoestrogens in cancer prevention and therapy–mechanisms of their biological activity. Anticancer Res. 2005; 25: 2357–66.
Affiliations
Amy L Strong1, Marjorie E Bateman1, Robert B Jones1, Maria F Dutreil1, Annie C Bowles1, Isadore M Budnick1, Shilong Zheng2, Daniel J Hayes3, Benjamin Levi4, Jeffrey M Gimble1,5, Matthew E Burow1, Guangdi Wang2, Margaret A McNulty3 & Bruce A Bunnell1*
1 Tulane University School of Medicine, New Orleans, LA 70112, USA
2 Department of Chemistry and RCMI Cancer Research Program, Xavier University of Louisiana, New Orleans, LA 70125, USA
3 Louisiana State University, Baton Rouge, LA 70803, USA
4 Department of Surgery, Division of Plastic Surgery, University of Michigan, Ann Arbor, Michigan, USA
5 LaCell LLC, New Orleans, LA 70112, USA
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.

