Raw materials for cell & gene therapy: exploring regulatory and supply issues
Cell Gene Therapy Insights 2017; 3(3), 183-187.
10.18609/cgti.2017.024
The cell and gene therapy sector has seen a great deal of growth and progress over the last couple of years. What developments have interested you and why?
What stood out for us is the rapid expansion and maturation of the field as more companies are approaching late-stage clinical development and market authorization. As a result we’ve seen a lot of progress in accomplishing consistent manufacturing on a global scale. One of the biggest challenges in achieving this is the elimination of variations, especially in starting and raw materials (in the USA raw materials are called ancillary materials) and manual manufacturing steps. To eliminate variations in raw materials we see an increased demand for GMP-grade raw materials and an increased need for regulatory guidance. As a provider of GMP-grade raw materials we are actively participating in raising awareness of the need to use high quality GMP-grade materials and are in constant interaction with regulatory authorities worldwide. To overcome variations due to manual manufacturing steps, a lot of progress has been made in developing automated solutions. Automated manufacturing examples that have impressed us most are the cell processing systems developed by companies like Octane and Invetech. Their solutions manage to combine different cell processing steps in one closed system for the production of autologous and allogenic treatments. The transition to automated manufacturing of major products will augment the need for high quality raw materials in even larger quantities. We are very interested to see how this will also impact the need for customized raw materials. In certain production settings, it would be advantageous to have, for example, medium in a special bag or cytokines in different forms (e.g., larger fill sizes, bulk material, mixture of cytokines).
The regulatory agencies are starting to acknowledge the increasing need for guidance around the quality of raw materials but there still seems to be a degree of confusion within the sector – why is this?
In our opinion, the current guidelines do not offer the level of guidance that is needed. With USP Chapter <1043>, the FDA only offers a recommendation for raw materials users and not for the manufacturers of raw materials. The EMA’s new Ph. Eur. General Chapter 5.2.12 is rather unspecific and can be interpreted in different ways. A more clearly specified guideline would simplify the harmonization of quality and safety requirements. The challenge is, however, to write one guideline for all raw materials since there is a large diversity between the raw materials being used in cell and gene therapy manufacturing. In addition, there’s currently no global harmonization although we do see a strong push from the market to change this. To help improve regulatory guidance we are actively involved in many of the regulatory initiatives and discussions. Together with the USP we have written the first version of USP <92> and were actively involved in the discussions for the setup of Ph. Eur. General Chapter 5.2.12. At the moment we are actively contributing to the ISO technical committee TC276. This committee is the only global initiative that is working on a best practice guide for raw materials for suppliers and for users. The guide is aimed to be the global ISO technical standard.
What do you see as the core quality requirements needed to reduce concerns regarding product quality and safety?
We think the following three core quality requirements are needed:
Raw materials must be safe. The origin and impurity profile of all materials used for the manufacture of GMP-grade raw materials should be assessed, including an assesment of all materials for the presence of animal-derived components in their respective manufacturing. They should be procured from reliable manufacturers and suppliers. Whenever possible, the use of animal- or human-derived components should be avoided and only safe and traceable materials must be used throughout the raw material manufacturing process.
Raw materials should be manufactured following applicable GMP guidelines to provide documented evidence of purity, potency, consistency and stability:
- Manufacturing and QC according to Standard Operating Procedures (SOPs)
- Qualified and trained personnel
- High class clean room facility and qualified equipment
- Validated and consistent processes (manufacturing, cleaning, QC methods)
- Monitoring of product quality by in-process controls (IPC) at each manufacturing step
- Product release according to pre-defined specifications by a QC completely independent from manufacturing
Raw materials must comply with regulatory requirements:
- USP <1043>, USP <92> and Ph. Eur. General Chapter 5.2.12.
- ISO 9001:2008 certified Quality Assurance (QA) system, which is constantly improved and regularly inspected by certified bodies (ISO 9001:2015 is coming soon).
- Manufacturing in compliance with relevant GMP guidelines, including key GMP demands such as change control, Out of Specification (OOS) procedure, etc.
- Analytical methods are based upon relevant International Council for Harmonization (ICH) quality guidelines.
Overall, we believe that the use of GMP-grade and animal-derived component-free (ADCF) manufactured raw materials, precured from safe and traceable sources, will significantly reduce qualification and validation efforts of cell and gene therapy manufacturers.
Scale up is one of the key challenges faced by companies in this sector. What advice would you provide to support these transitional steps in terms of raw material scale up?
Scaling up from clinical trials in Phase 1 to large-scale commercial manufacturing the number of patients (i.e., doses to be produced) might go up by several magnitudes. As a consequence the supply of high quality GMP-grade raw materials is getting more critical. If high quality GMP-grade raw materials are not available in time this could lead to delays in cell and gene therapy production. This does not only increase costs but also puts precious patient samples in jeopardy. A raw material supplier must, therefore, be able to provide materials in strongly increasing quantities in a consistent high quality. Their manufacturing processes must be appropriately designed in order to allow for a scale up as needed.
The safest way to eliminate possible problems with the supply of GMP-grade raw materials is having a supply agreement in place well before large-scale manufacturing is planned to start. Giving the supplier a clear and reliable forecast of doses to be produced and consequently the amounts of raw material needed will allow the supplier to have enough products available on demand. As the supplier, this for instance allows us to produce large size batches that are reserved for one customer.
In case unique bio-processing models are used for cell and gene therapy manufacturing, customized GMP-grade raw materials might be desired. For customized products the supply chain gets even more complex. The initial setup and full validation process of a custom product under GMP requires a good amount of time. If full stability of the final product has to be demonstrated, more than a year might be needed from initial design to delivery of the first batch. We therefore recommend getting a clear understanding on time needed from design to delivery of these customized products well in advance.
At what stage do you typically see cell and gene therapy companies starting to think about using GMP-grade materials in their processes?
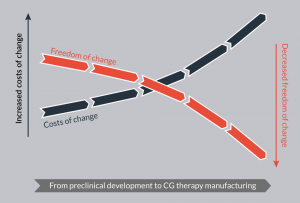
Figure 1: Impact of change on manufacturing costs depending on the stage of development of a cell and/or gene therapy.
We see a large variation in customer awareness regarding when they should start thinking about using GMP-grade raw materials. Companies that have large clinical studies planned think about it earlier since they need to consider things like security of supply and late-stage cost calculations. We also see that companies that are new to the field learned from the early-market ‘mistakes’ and start to think about GMP earlier in the process.
To enable a seamless transition we recommend identifying the appropriate GMP-grade raw materials and reliable suppliers already during early stage preclinical research and prior to clinical development of the cell and gene therapy. Changing raw materials at a late stage in clinical development in order to meet regulatory requirements often creates significant additional costs and loss of precious time. These additional costs are only to a small extend caused by the increased costs of GMP-grade raw materials. They are primarily related to the need to perform clinical comparability studies. Switching to the required GMP-grade raw materials at an early stage will prevent the need for such studies and thereby brings a significant economic benefit. A study performed by the Tufts Center for the Study of Drug Development (CSDD) estimated that the costs of an amendment for a Phase 3 trial costs more than three times as much as an amendment for a Phase 2 trial [1]. They estimated the average direct cost to make changes to the protocols of Phase 3 trials tops $1 million per study (Figure 1).
There’s often discussion of the need to move to animal-derived component-free materials in the CG supply chain. How challenging is this and why is it important in cell and gene therapy manufacturing?
We see several reasons why it’s important to use animal-derived component-free (ADCF) materials. The most important and obvious one is to ensure the safety of the cell and gene therapy being manufactured. Furthermore, animal-derived components such as serum are heterogeneous and very expensive. They therefore have a negative effect on product consistency and cost effectiveness of the cell and gene therapy.
The challenge is to substitute critical animal-derived components by chemically defined ones that have the same functionality at acceptable costs. For recombinant proteins we are very successful in doing this. However, it still forms a challenge for the serum-free culture of certain cell types. This is caused by the fact that the composition and functionality of human serum is not fully understood yet, making it difficult to find a chemically defined substitute that is as effective as human serum.
We are continuously looking for ways to further improve the ADCF level of our products, as an example we have implemented a strict segregation between our dedicated animal-free facility and our production area where human cell lines and animal-derived materials are allowed to enter. Furthermore, we are continuously improving our media formulations by the substitution of critical raw materials by safer and more consistent ones.
Looking forward, worldwide harmonization of guidelines for raw materials will be one of the key drivers to allow for decentralized or proof-of-concept manufacturing around the globe. Using the identical raw materials in different production locations in different countries facilitates the easy access to these critical production materials from one source, eliminating the need to do expensive and time consumable comparison studies for different production sites.
Reference
Need to Make Changes to Your Clinical Trial? It’ll Cost Money and Time. Website
Affiliation
Dr Bernd Leistler
Vice President Development & Production, CellGenix GmbH
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.