Factors influencing automation decisions in cell & advanced therapy manufacture
Cell Gene Therapy Insights 2016; 2(4), 481-487.
10.18609/cgti.2016.055

Brian Hanrahan has more than 15 years of product development experience across the biomedical and cell therapy industries. In his current role, Brian is the Manager of Invetech’s Cell Therapy Group in San Diego, CA, USA, helping cell and advanced therapy companies across the globe realize clinical and commercial-scale cGMP manufacturing solutions. Brian has been a key contributor in building Invetech’s cell and advanced therapy capabilities and continues to have a deep involvement in projects ranging from the development of cell separation instruments to single-use, automated cell therapy production systems. Brian has a bachelor’s degree in Applied Science from RMIT University in Australia.
What are you seeing as the main drivers for companies moving towards more automated manufacturing processes?
I think it’s important to first define what we mean by automation in the context of advanced cell and gene therapies. When you use the term automation, generally people have this image of a car manufacturing assembly line or a laboratory with high-throughput robotic stations, which really represents very sophisticated automation. Our approach to automation in relation to advanced cell and gene therapies is simpler and can be thought of a little differently: the critical element of automation being that it takes place in a closed single-use disposable, thus enabling the process to be run in a clean unclassified or lower grade clean space.
A key driver for this move towards automated, closed single-use disposable systems is of course cost savings. Automation generally is looked at as an effective way to reduce your manufacturing costs, and certainly where applied appropriately, it can help to significantly reduce the labor component of a manufacturing process with some companies seeing a 40–90% reduction in direct labor costs – but that’s not the sole rationale. Companies also want to ensure that they can manage and maintain the quality of their product. By automating steps in the process you can provide that quality assurance as the process is the same every time it’s run.
Are there any trends you are seeing with regards to when companies are starting to think about automation in their product development lifecycle?
We recently conducted a market research survey within the industry and found that over 80% of cell therapy companies are using manual laboratory processes in pre-clinical studies, with this dropping to around 50% by the time they move to Phase 1/2 clinical trials. Around 80% stated that they anticipate moving to an automated system as they progress towards commercial production.
From our perspective, when you are at Phase 1 development stage, you really want to be exploring ideas and developing your commercialization plan. As you start to build a clearer picture of your indication, determine the predicted number of patients/year, and decide whether to adopt a centralized or decentralized manufacturing model, then you need to start thinking about the impact that has on selecting and implementing the appropriate level of automation. Which of your unit processes are not going to be practical or cost-effective at commercial scale?
As you progress towards Phase 2, this is the ideal time to identify what technologies and processes can be appropriately automated and if necessary integrated into your manufacturing workflow. You might be looking at more of a semi-automated process initially, but using the technologies that you are going to utilize at commercial scale. Therefore, you will have a much clearer path for regulators to see that, while the process becomes more automated at commercial scale, it’s not fundamentally being altered and the product output is the same.
What is your approach to identifying which processes in a manufacturing workflow should be prioritized for automation?
We start by taking a look at every step in the manufacturing process and identifying those that are most variable. From there, we start to think about the timeline and technology to address potential problems. How can you make processes consistent? How can you adapt them to a closed manufacturing system? These decisions will often be based on the most complex steps in the manufacturing process. But you have to plan for changes in processes that present a risk of variation in the cells and also the “skilled steps” or complex manipulations that are handled manually initially.
In cases where manual processes do not affect quality, you may be able to delay decisions on automation. It is ok to continue to use manual operations as long as they do not increase risks or costs. For example, one of the more common and straightforward steps that we work with clients on is the washing of cells. In the research setting, this is a simple process often with conventional centrifugation steps that occur throughout the workflow. The requirement as you move towards commercial-scale manufacturing is to find an appropriate technology that allows you to do that wash and concentrate step but in a functionally closed system.
On the other end of the complexity spectrum is the automation of intricate, skilled processes. It’s these unique and complex steps in the manufacturing process that a company needs to look to automate for commercial production. These are typically the steps undertaken by a skilled operator, and often an open process, which is the last thing you want to be taking through into your commercial-scale manufacturing, particularly if you are in an autologous cell therapy setting with a large number of patient products to manage per year. It is the more complex interaction and manipulation steps that should be prioritized for automation in the first instance and they tend to stand out pretty clearly when we review a client’s processes.
Do you feel there’s a good understanding within cell and gene therapy companies of what is required in terms of data and reproducibility for regulatory approval?
I would say that most do have a good level of understanding. They recognize that from the regulatory standpoint, until we have more absolute product characterization, the product is the process. Therefore, changing a part of your process after Phase 3 will likely result in having to carry out another comparability study to prove those process changes haven’t altered the product.
We actually just published an interview series where we asked several cell and advanced therapy companies and industry leaders to share their perspectives on topics such as obtaining funding/financing, regulatory considerations, and scaling manufacturing. In the regulatory interview, representatives from Celyad, Argos Therapeutics, and Humacyte shared their insights about reproducibility. They all expressed the importance of being able to manufacture product in a consistent and reproducible way and understand the implications variability can have in Phase 2 clinical trials. In one of the interviews, Bill Tente, Vice President of Quality, Compliance Management & Regulatory Affairs at Humacyte, referenced a company that introduced a manufacturing change in a pivotal randomized trial that had a significant effect on the biological characteristics of the product. The impact on timeline and costs forced them out of business. Although he acknowledged that might be an extreme example, it shows that changes at any stage can increase the risk of delays or a clinical hold that can be devastating.
As with anything, when you become more involved in the details, that’s when it becomes more obvious as to where opportunities for variability lie within a process. While there is often some need for education there, by and large our clients generally recognize what is required in terms of data and reproducibility as we explore automation solutions with them.
What are the perceived barriers to implementing automated processes within cell and gene manufacturing?
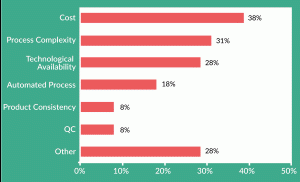
Figure 1. Perceived barriers to implementing automated manufacturing for cell-based therapies. N = 39. Quesiton: “What do you consider to be the greatest barriers to implementing fully automated manufacture of cell-based therapies?”
As part of the market research survey I mentioned earlier, we asked about the biggest barriers to implementing fully automated manufacturing solutions and the top two answers were costs and process complexity. This presents a very interesting conundrum because they are actually two of the main reasons you would move to automated processes (Figure 1). But it’s understandable that companies look at their processes and assume that the move to automation is just going to be too complex and add costs to their manufacturing. However, as you start to analyze your processes in a more detailed way and you look for where the costs are distributed, it becomes much clearer that in fact introducing automated technologies and processes can not only simplify these steps but bring down your costs at the same time.
One example I would like to share here is our experience in helping a company scale-up an allogeneic process and being able to validate that process. This particular process was a 2D adherent culture and when the disposable system was filled with cell suspension it was around 15 Kg in weight. If you think about the practicality of carrying out manipulation steps such as cell harvest and trypsinization at this scale in a manual fashion, it’s clear that you are going to encounter a lot of challenges, not only physically, but also in ensuring you are carrying out these steps in a consistent way each time. But as you translate this into an automated process, you can replicate these manipulation steps at scale, consistently. That’s a very powerful driver – and actually a relatively simple level of automation would be required here and yet it would transform your process into one that can be validated.
A second example is a cell seeding operation that for early research work uses two operators manually seeding cells (20– 50 ml) onto a biodegradable scaffold within a 500 ml container and the process takes 30 minutes. When you scale-up and want to seed several hundred concurrently, this creates a large labor requirement that’s just not cost-effective or logistically practical. The solution to automate this process may be costly, but we have found that the ROI for these solutions can be realized in less than a year, even when the implementation (development and replication) cost is significant. Prior to implementing any solution, you need to explore the technologies available and the impact these solutions can have on the cost of goods.
A variety of different manufacturing processes are being utilized by companies even when using the same cell type. Is it therefore realistic to try and provide off-the-shelf (OTS)options for automation?
When talking about OTS automation options, it’s important to think about integration and how that integration will look at commercial scale. For example, if you integrate all unit processes into one system and you have a cell expansion (incubation) step that takes more than a week, then the entire system would be locked down for long periods of time. While this might be manageable in Phase 1/2 when you have small numbers of patients, your ability to optimize utilization of capital equipment becomes much more critical at commercial scale with hundreds of patients.
It comes back to really understanding what your processes need to look like as you move towards commercialization. For example, if you are working with an autologous therapy, one of the crucial questions would be ‘how many patient samples are you going to be handling in a given timeframe?’ If you don’t have a sense of what that number is, then it’s very difficult to know whether these OTS automation solutions can be integrated in a way that simplifies the overall workflow, reduces operator requirements, and optimizes costs at commercial scale.
That being said, there are quite a few OTS options available for some of the key unit processes. Taking the example I mentioned previously, the wash and concentrate step. There are a number of solutions available now that enable you to use a functionally closed process in which to wash and concentrate your cell suspension. If we look at the expansion step however, where we are working with 2D expansion, cell factories, etc., there’s still a bit of a gap in terms of developing suspension cultures in bioreactors – whether that’s adherent expansion in which you are using micro-carriers or cell expansion in suspension culture that are not adherent.
On the other hand, for companies that have really complex or unusual unit processes within their manufacturing workflow, it will be very difficult to get an OTS automation solution. In such cases, they will likely need to explore how they can implement an appropriate customized solution – especially when that unit process is core to the product quality.
There is a great deal of discussion regarding the need to reduce manufacturing costs if we are to develop commercially viable products. What role can automation play in achieving a reduction in the costs of goods?
One of the main opportunities for reducing the cost of goods for an autologous cell and gene therapy is through automation that allows the use of functionally closed, single-use disposables and operating in low-grade clean room space. If the company invests heavily in their manufacturing facilities but hasn’t thoroughly envisaged a commercial-scale process in lower-grade clean room space, then they can be unnecessarily driving up their cost of goods. We see that there’s around a five-fold cost difference between leasing the required Class B (manual) and C (closed) space. Savings on owner-occupied facilities are even greater. So very quickly you can see how important it is in autologous therapy manufacturing to adopt single-use disposables and to have fully automated and integrated processes.
Within the allogeneic setting, it’s a different situation. You are scaling up rather than scaling out, so it’s more of a focus on how big a volume you can get a batch to be and how you can guarantee product consistency as you move to larger batches. That closely follows the Big Pharma drug development and manufacturing approach.
A number of players such as Juno and bluebird bio are establishing manufacturing capabilities in-house. What do you see as the driver for this trend and do you expect the sector to follow the Big Pharma model over time whereby manufacturing becomes more outsourced?
Many companies in the cell and advanced therapy space feel that working with a contract manufacturing organization (CMO) is a good initial start because they have the infrastructure in place to get them started. As for the examples of Juno and bluebird bio, I think that there comes a point when you want to maintain tight control of your processes and therefore we’re seeing this shift towards bringing manufacturing in-house as a way of achieving that. Another factor that is no doubt part of the conversation here is whether CMOs are set up to manufacturer some of these higher volume therapies at commercial scale.
One piece of advice I would give, is that you need to prove the therapy works before you start building out large facilities. A fairly common planning mistake is investing too much too soon by over-estimating your production needs and developing much larger facilities than are actually required at the outset.
Where you do feel the opportunities for innovation lie within automation and manufacturing?
If you go back 5 to 10 years, the industry was grappling with the logistical question of fresh product versus frozen for autologous therapies. In an ideal world, we would prefer to have fresh product because it would remove the issue of reduced cell viability caused by having a front-end cryopreservation step for your initial cell material, and then another cryopreservation step at the end of the process for the final product. The logistical benefits of one or two freeze–thaw steps were compelling. Now we are starting to revisit this assumption as we look towards new options for near-patient processing and manufacturing where advances in technology, communication and data management are really improving our ability to handle fresh products.
As we look ahead at the manufacturing advances that are being made in a variety of different areas, it becomes more of an option for an autologous product to be made potentially near patients. A good analogy here is in the diagnostics space where you now have point of care instrumentation that can perform the diagnostic with the patient on-site in 10 to 15 minutes. These tests were previously performed in a diagnostics laboratory where the sample had to be transported to the lab and the turnaround time for the result would be at lease 60 minutes. Within the cell and gene therapy field, more often we are starting to ask: does this process need to be performed in a cGMP manufacturing facility or can I manage that process much closer to the patient in a cost effective way? It will be interesting to see how this side of manufacturing continues to evolve over the next few years.
Affiliation
Brian Hanrahan, Program Manager, Cell Therapy, Invetech, USA


This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.