The key to commercial success: process development & logistical considerations for autologous immunotherapies
Cell Gene Therapy Insights 2016; 2(4), 453-461.
10.18609/cgti.2016.051
The success of a cell therapy clinical trial rests on the ability to deliver a viable, potent product. This positive end result is directly attributable to the strategy in place and the supporting processes. A reliable cell therapy logistics strategy is imperative in ensuring your biopharmaceutical products, therapeutic materials and patient biological samples remain viable from the point of manufacture to the final clinical site delivery.
The success of a cell therapy clinical trial rests on the ability to deliver a viable, potent product. This positive end result is directly attributable to the strategy in place and the supporting processes. A reliable cell therapy logistics strategy is imperative in ensuring your biopharmaceutical products, therapeutic materials and patient biological samples remain viable from the point of manufacture to the final clinical site delivery.
Among the basics of building a successful logistics strategy for the management of cell-based material, some better-known and important factors to consider include: selecting the right dry-shipping unit, qualifying that container for a particular payload and shipping configuration, choosing an appropriate data logger, creating a chain of custody, evaluating a transit carrier and anticipating potential problems inherent in shipping at cryogenic temperatures [1]. Here, I’d like to go beyond those basics to address some lesser-known considerations. These factors may be hidden in the background, but they can play a major role in the success or failure of a clinical trial as well as the long-term efficacy of a cell-based commercial product. They include standardization, package and shipping qualification, equipment validation, process qualification and documenting the chain of custody.
Several unique logistical challenges arise for autologous cell therapies-those that use a patient’s own cells for the manufacture of a treatment that is then administered to that patient only. By contrast, allogeneic cell therapies are derived from unrelated donor(s) and administered to a relevant population of patients. All of these considerations apply to both types of cellular therapies.
The unique complexity of autologous therapy
Figure 1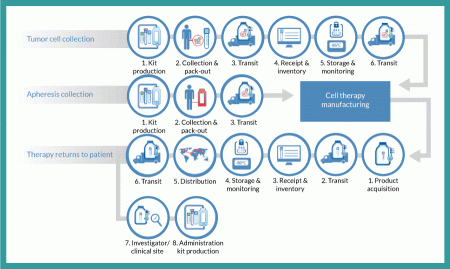
Tumor cell collection
From a logistics perspective, the entire autologous cell therapy drug-production process begins with creation of a kit for collecting tumor cells and securely identifying the resulting sample with a specific patient. A unique identification number is assigned that will be used throughout the entire process of collection, manufacturing, distribution and administration. Such procedures ensure that the right product is infused into the right patient. This tumor-collection kit will include a qualified shipper for transporting tissue to an interim storage point. Using this kit, clinical staff will collect a tumor sample and package it for transport (by a common carrier) to a storage facility, where it will be received, inventoried and stored until ordered for manufacturing. At that point, it will again be packaged in a qualified shipping unit and transported to the point of manufacture, where it is stored until manufacturing begins.
Apheresis collection
Timing and logistics are critical with regard to apheresis collection because an autologous cell therapy manufacturing process begins in earnest upon receipt of the resulting dendritic cells. Unlike the tumor-collection process, no interim storage step interrupts the progress of this sample. As with the tumor collection, however, the process begins with a kit that includes all components required for collection. This kit includes patient-specific identification labels and collection containers as well as a qualified shipping container. Dendritic cells are shipped by a common carrier with the addition of an important step: notification of the manufacturer that the shipment is on the way. The resulting shipment is received by the manufacturer, which confirms the patient identity and begins the manufacturing process.
Therapy returns to patient
Once manufactured, autologous cell therapy doses are cryopreserved and loaded into a qualified dry-shipping unit for transport by a common carrier to a distribution facility, where the material is received and inventoried. When that therapy is packaged to leave the manufacturing site, it is ‘acquired’ for distribution and begins the journey of a drug to a patient. At the distribution center, individual doses are inventoried and stored in a vapor-phase nitrogen freezer until requested by a clinical investigator or physician for patient use. Each requested dose is shipped in a qualified dry-shipping unit by common carrier to the clinical site for administration.
Standardization
It is important to standardize as many processes and procedures as possible in the development of a logistics strategy. Some processes will remain outside your direct control. For cell collection, drug preparation/processing and product administration, kits can be a very effective tool by which a company can define materials needed and drive consistency in the execution of these processes. Figure 3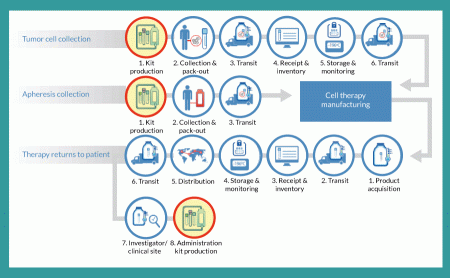

The value of such kits as standardization tools increases as a cell therapy candidate traverses the advanced clinical trial phases. Early stage (Phase 1 and 2a) trials often involve only one or two clinical sites. That limited number of sites allows for close coordination with a product developer to ensure that materials handling is coordinated and consistent.
But a challenge arises as such products move through Phase 2b and 3 trials. Those studies often include 20–50 clinical trial sites, so it becomes far more difficult to ensure that each one follows the exact same procedures for cell collection, product preparation and administration. One key means of standardizing those processes is to have the clinics use specially designed kits for cell harvesting (collection kits) and for delivering finished drug doses to patients (administration kits).
The use of a kit ensures that the same materials will be used in each process, that instructions are available for each procedure, that labeling is consistent and (in the case of collection kits) that a qualified shipping solution is used for transporting critical patient cells following their collection. The degree to which premade kits affect the outcome of a clinical trial depends on the complexity of the process they address. Providing an administration kit for a product that requires surgical implantation is far more critical than for one that is introduced through intravenous administration. In every case, however, the goal is to reduce process variation and ensure that clinical results reflect the efficacy of a given therapy rather than variations in its handling.
Package and shipping qualification
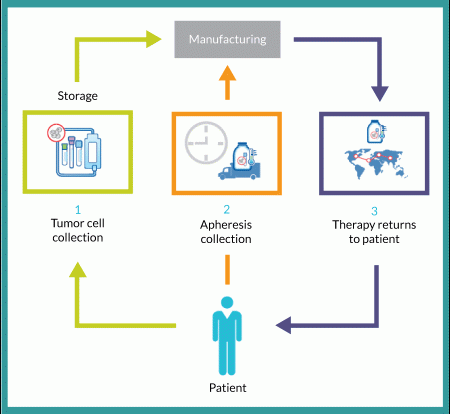
Successful transit of material is a primary objective of any logistics strategy. Consider that the simple flow diagram in Figure 1 contains five different shipments of three different materials at four different temperatures:
1. A patient’s tumor (or other applicable tissue) is collected and shipped at controlled ambient temperature to a storage facility for preservation at -80 °C.
2. When needed, that patient’s tissue is then shipped on dry ice to the cell therapy manufacturing facility.
3. The same patient’s apheresis collection is shipped under refrigeration (2–8 °C) directly to the manufacturing facility.
4. Finished therapeutic doses are then shipped in bulk from the manufacturing facility to a storage/distribution point using dry-shipping containers at cryogenic temperatures.
5. Doses are then shipped (again in dry-shipping units) a final time to the clinical site for patient administration.
For each of those five transit points, it is critical to use qualified shipping solutions that are designed to meet the specific requirements of each individual payload for temperature, duration and environmental and handling extremes that may be encountered during transit. Those specifications are not to be confused with the blanket qualifications often issued by the manufacturers of shipping solutions. Such information can be useful in guiding your choice of possible solutions for testing. But they fall short of the rigorous testing needed to ensure that material being shipped will reach its destination in perfect condition.
A qualified shipping solution is critical to ensure that your cell therapy is judged on its clinical efficacy rather than based on deficiencies created by packaging or shipping failures. Figure 5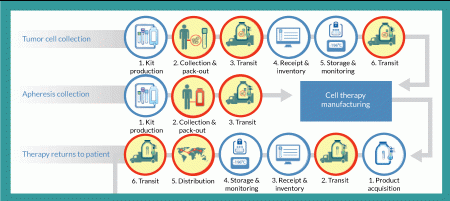
It is imperative that each individual dry-shipping container be tested because the units can vary significantly. This is not the case only for different makes and models, but also for gauging performance of shippers from the same model and lot. Figure 6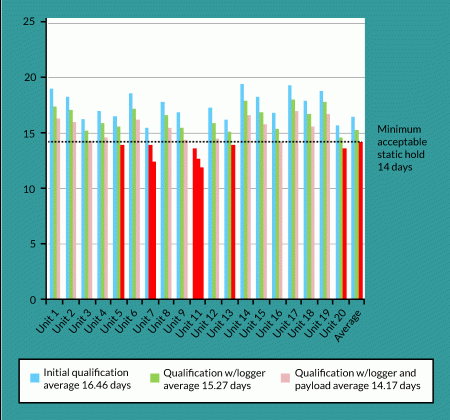
Beyond the shipping unit itself, three key variables influence the amount of temperature hold time required for each shipment. The first is the time it takes for an international shipment to clear customs. In most cases, customs can be cleared in 24–48 hours, but in some countries that can take considerably longer. Clearance times vary not only by country, but also with the volume being processed on a given day, delays caused by documentation problems, local holidays, weekends and inexperience on the part of a given agent, just to mention a few possibilities. Companies must calculate a safety margin into their products’ temperature-hold requirements.
Second, dry-shipping units are often used for temporary storage at the sites of patient administration. How much time will be needed to prepare and administer a given therapy to the patient once it has arrived at the clinical site? Finally, you need to consider the amount of temperature hold that can be lost in mishandling. Dry-shipping units are designed to be used in an upright orientation. Any deviation from vertical will rob the unit of valuable hold time. For example, a unit that is tipped over on its side for 8 hours can lose as much as half of its hold-time capability. Given these variables, it is important that you choose dry-shipping units that allow for the longest hold times reasonable and that you validate them under both static and dynamic conditions (Figure 1).
Although shipping of cryopreserved materials may be the most extreme logistical challenge, it is equally important to ensure that all biologics are shipped in qualified containers designed to meet the material’s temperature and duration requirements. The key to success is finding a solution that meets temperature and duration requirements in a procedure that can be easily implemented by the individuals who will perform the pack-out procedure. In late-stage trials, that may happen in hundreds of locations involving people with limited experience in the transport of such materials. So the best solutions are those that are least complicated; the more complex a process, the more likely it is to have deviations and failures. Unfortunately, the easiest solutions are often the most costly, so you will need to evaluate cost against the risk of loss for each shipping requirement.
It is also important to consider where and when you are shipping your materials. A qualified solution that works in Maine in January may not work in Arizona in August. Among several available options, the two most common include creating summer and winter shipping profiles and creating a universal shipping solution. Establishing summer and winter profiles is generally the most cost-effective approach, but doing so can require tricky decision-making for changing seasons and weather patterns. A universal solution can be more costly, but it will be considerably easier to manage.
Volume is the final consideration. Autologous cell therapies are unique in that human cells are their primary active pharmaceutical ingredient and must be harvested, shipped and incorporated into the drug-manufacturing process within a very tight window of time. That is generally a manageable process during clinical testing, but it becomes a monumental logistical challenge for commercial operations. In autologous production, the manufacturing process begins when cells are harvested. So you will need an effective scheduling and tracking system and a dependable way to transport viable materials. There is no way to manage production without tight scheduling and tracking processes in place. It is wise to begin laying ground for both during Phase 2 studies, and it will be imperative for you to test commercial-volume solutions by Phase 3 (Figure 7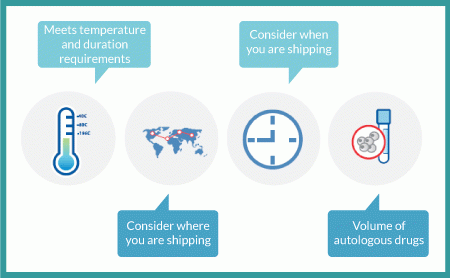
Storage equipment validation
Validation of equipment used to maintain such valuable materials is an absolute requirement at every stage. Just as the shipping units can vary in performance and must be qualified, so too can storage equipment. It must also be validated to perform in the specific way that it will be used relative to the materials involved. Figure 8
Process qualification
In much the same way that we validate equipment to ensure that it will meet requirements, we must also test the procedures used for materials handling. For any point at which temperature-sensitive material is physically handled, a process qualification should be considered. Figure 9 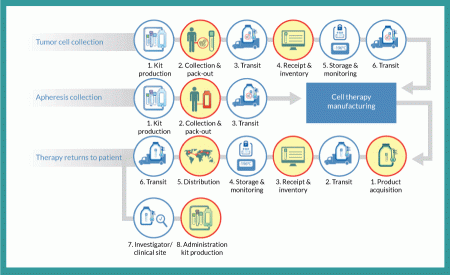
It is also important to consider the cumulative effects of temperature variations on your product. For example, with a product stored in cryovials, assume that those vials are stored 50 units to a box and stacked four boxes to a rack. Each time a dose is removed from storage, that rack is moved into an ambient environment. All vials in the rack – not just the one removed–are briefly exposed to a (short-duration) temperature excursion (Figure 10
Whereas a great deal of effort is often expended in determining the effect of a single temperature excursion, far less attention is paid to the effects of cumulative exposure. In the example above, the first dose is exposed only once, but the last dose may be exposed 200 times. Although the actual handling times vary by product, it is good practice to develop processes that minimize product exposure and then to validate those processes to ensure that they do indeed meet your specifications.
Chain-of-custody documentation
With all cell therapy drug products, a chain of custody begins at the point of manufacture and concludes with administration to a patient. Autologous drugs require that chain to extend backward to collection of the constituent cells used for drug production. In all cases, chain-of-custody documentation must capture the location, security and temperature of materials at all times. That chain of custody encompasses every process step in Figure 1.
Given that a chain is only as strong as its weakest link, documenting the chain of custody necessitates that all processes be given equal weight with regard to collecting and documenting accurate and complete data. A loss of documentation at any point renders the entire chain inadequate. Maintaining suitable documentation of a custody chain can be far more difficult than it would seem. Because processes occur in different settings and are performed by multiple individuals at different organizations, a robust procedure must be in place to ensure that the requisite data are compiled and collected in a standardized way. The resulting documentation must be maintained at a per-dose level and be readily available for review whenever necessary. And special consideration must be given to the last link in this chain: the point of patient administration. The clinical site – where the final leg of storage, processing and administration occurs – is invariably the most difficult area for consistent collection of necessary documentation.
Plan ahead for success
All these logistical challenges should give you pause. Every company wants its cell therapy to be successful. By informing you well in advance about issues you are likely to face in delivering a cell therapy to patients, I hope to help you prevent unnecessary delays and costs as you progress. An experienced logistical partner can help smooth the entire round trip of an autologous cell therapy: from initial collection of cells to delivery of a finished dose back to the waiting patient who needs it.
Affiliations
Dan H O’Donnell
Director Cell Therapy Logistics, Fisher BioServices

This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.