Automation in the context of stem cell production – where are we heading with Industry 4.0?
Cell Gene Therapy Insights 2016; 2(4), 499-506.
10.18609/cgti.2016.060
As the field of cell and gene therapy continues to progress, the need for cell products for therapeutic application is increasing. In order to meet the demands, novel challenges particularly within the manufacturing of these products need to be overcome. By translating and applying automation solutions from the production industries into cell culture applications, standardized processing procedures can be introduced in order to reduce the variability, which is a critical issue within the manufacturing workflow. Additionally, the Industry 4.0 philosophy offers a variety of concepts for the design of adaptive processes that address the specific challenges in stem cell production. Novel tools such as data tracking, data analysis and machine learning are enabling technologies that will help support the safe manufacturing of effective products for future cell and gene therapies.
Manufacturing Challenges for Therapeutic Cell Products
In recent years, there has been significant clinical and commercial interest in generating cell material for therapeutic applications. The major reason is the advances in stem cell research and the progressing clinical studies revealing the potential of stem cells for regenerative therapy [1]. In order to fulfill clinical demand however, there is a need for reproducible and robust manufacturing processes. In the case of some stem cell therapies, it is estimated that approximately 7 x 107 cells are needed per dose for a patient with a body weight of 70 kg [2,3]. Additionally, it is likely that for some applications, multiple doses will be required. In order to meet the growing need for high quality cell products however, a number of novel challenges have to be addressed.
In contrast to conventional biopharmaceuticals produced by industrial cell cultures, the product comprises the living cell itself which bears a certain inherent complexity and variability [4,5]. An additional complication that arises and must be taken into account for patient-specific processes is the impact of donor-to-donor variability [6]. As opposed to traditional industrial processes, where well characterized cell lines are utilized, here it cannot be drawn from extensive process know-how. Instead, cell behavior and optimal cultivation conditions can differ significantly between two batches. This effect is intensified when manual labor is employed to expand the cells due to variations in the handling procedures that can only to some extent be reduced by the application of detailed operating procedures.
These combined factors result in a prevailing risk that two batches of the final product may differ in their properties and efficacy. This situation is further intensified by the current lack of suitable tools for stem cell characterization [7]. This however contradicts the imperative of having well defined, reproducible medical products, which is necessary in order to guarantee a safe and efficient medical application. Moreover, using standardized cell products is central to obtaining reliable and reproducible results. Surveys have shown that providing consistent cell qualities to standardized conditions remains a major concern for manufacturing companies [8].
Confronted with this situation, manufacturers for cell products have to find solutions in order to pave the way for cell and gene therapies. Whilst this, in part, dictates a research focus to improve process and product understanding, there is also a regulatory driver to consider the integration of technical solutions such as automation, which are likely to play a fundamental role in the development of innovative and reliable manufacturing processes.
Translation of Tools from Production Technology
Production of pharmaceutical and biopharmaceutical products has traditionally relied on technical systems such as bioreactors for cell manufacture. In recent years, the trend has moved towards an increasing level of automation. In comparison to other production sectors such as automotive and aviation industries however, biotechnology is still lagging behind. In high-wage countries in particular, automation has been one of the main key factors enabling a reduction in production costs. In addition to potentially reducing cost of goods, automation also offers enhanced reproducibility, reliability and increased throughput.
In contrast to industrial production however, biological processes differ in complexity and variability. With production technologies moving towards process agility, there are many elements of the current technologies that can be transferred to biomanufacturing applications. For example, the requirements for the cultivation of primary cells from varying sources are similar to the challenges found in the individualized production of single pieces with limited automated resources. With the attention shifting from lab-scale to large-scale production, cell manufacturing for regenerative medicine can benefit from automation by combining robustness and process adaptivity.
It is also important to consider the advances in digitalization of machines and processes, which no doubt offer new possibilities for data-driven and adaptive production. In this context, the term ‘Industry 4.0’ is often used to summarize these developments. Although this term can be difficult to define, there are essentially nine aspects associated with Industry 4.0: interconnection, collaboration, standards, security, data analytics, information, decentralized decisions, physical and virtual assistance [9].
In the following sections we describe how production engineering concepts can be translated in order to address the challenges arising within the manufacturing of therapeutic cell products. Reproducibility and standardization can be achieved by collaboration of various automated devices and sophisticated data analytics. To enhance the reliability of technical systems, standardized control hardware in combination with high-grade information acquired from multiple sensors can be utilized. By using decentralized decisions and an interconnectivity of devices a high degree of adaptivity can be achieved. The combination of traditional automation and Industry 4.0 has the potential to achieve safe and well defined production of cell products for therapies in the future.
Reproducibility & standardization
As mentioned, in general there are two different sources of quality fluctuations in cell culture products. The first results from a natural variability in donor cells/tissue. The second source arises from variations in the production process. Although standard operation procedures are designed to describe a process as distinctly as possible, some of the handling tasks will always be executed with variations by different human laboratory operators. For example, pipetting speed and accuracy can vary depending on the operator and pipetting device.
To overcome these challenges automation can be a powerful tool. Classic industrial production focusses on stable and robust processes which are usually executed by automated machines and devices. By translation of these automation concepts into cell production, deviations in the processing steps can be significantly reduced.
For laboratory automation, various state-of-the-art devices can be transferred directly into cell production. For example, liquid-handling robots (Figure 1) can achieve a high degree of precision with pipetting steps by controlling parameters such as volume and pressure inside each pipetting tip. Using an automated system the accuracy of pipetting steps is often at one microliter or less. Moreover, most laboratory equipment such as incubators or centrifuges are often commercially available with an automated configuration. In order to interconnect the different devices physically, handling tasks can be performed by robot arms that can execute complex handling steps at a high accuracy and repeatability of movement.
In addition to the automation of handling and treatment steps, further benefits can be achieved within data processing. One example is the automated determination of the confluence, which is a typical parameter used for the assessment of 2D cultures. In manual culture, the estimation of confluence is subjective and depends on the operator’s experience and the microscope set-up, making it difficult to enable comparability between different operators. Here, cell culture analysis can benefit from automated image acquisition and subsequent image processing for automated confluence detection [10]. This enables one to introduce 100% quality control and carry out objective culture assessment that is reproducible.
Reliability of technical systems
In addition to accuracy, process robustness is always a major concern in production technology. For the characterization of production processes, process capability indices are commonly used criteria. More and more production branches aim for a 6 Sigma Level which translates to a maximal allowed error of 3.4 ppm. To reach these quality standards a high grade of process robustness is essential.
However, this demand remains widely unmet by the suppliers of commercially available laboratory automation devices. These systems are usually controlled by a standard computer with common operation systems such as Microsoft Windows. Issues such as software updates, risk of computer viruses, internal operation system errors and unexpected interruptions result in a low robustness of these control software.
In order to achieve a high degree of robustness in fully automated platforms, the implementation of Programmable Logic Control (PLC) is a key factor. The PLC allows for a real time processing and a robust functionality. This results in a high stability of the system which allows the running of machines and devices over decades. This approach is favorable for applications where the emphasis is on implementation of robust and rigid production processes.
Traceability
In the generation of cell products for medical applications, GMP requirements have to be met. Amongst other aspects, this has implications with respect to the documentation requirements, which often generate significant amounts of paper work with every step, decision and outcome being accurately documented and stored. Although changes in the process protocol have to be protocolled and justified, the degree to which things can be documented is limited. Minor changes such as deviations in the incubation time are often not traceable since they are not precisely monitored. Additionally, even if measures are in place such as controlling and double-checking, the risk of errors due to human failure can never be completely eliminated.
In the context of documentation and failure reduction, automation is a natural ally. By integrating all devices via standardized interfaces, complete traceability of all process steps, parameters and measurement data is possible. In addition, automation offers a significantly higher level of data generation and tracking than would be possible in a manual processes. This allows for the generation of a single dataset for each product batch which is continuously updated with all data associated with the product. The allocated information is not limited to measurement data or timestamps for the individual steps but also process parameters and comprehensive time courses of cultivation conditions such as the temperature in the incubator and pipetting accuracy.
The generation of such single datasets into which all data is integrated is often understood as a virtual model of the individual product, the so-called ‘digital twin’. This is another key paradigm of Industry 4.0, which in turns provides software tools and data architecture for its implementation. By integrating this approach, it is possible to trace the product along the complete product life cycle and also adresses the legal and regulatory requirements that apply to all medical products in their entirety.
Adaptivity
Whilst automation offers many advantages in terms of standardization and process robustness, in the past automated systems have often been characterized by their lack of flexibility. In contrast to the manual process where trained laboratory personnel can adjust the process steps – such as determination of the splitting time point – most automated systems offer only rigid protocols. Cell cultures on the other hand are often complex and especially when primary cells are cultured the processing of the cells needs to correspond to their growth behavior. However, this level of flexibility cannot be achieved using conventional automation concepts.
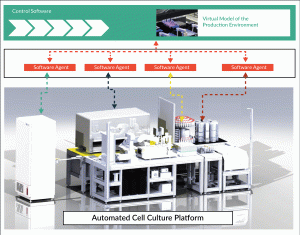
Fortunately, Industry 4.0 delivers tools that can respond to high product variability. In this case, the production platform can be organized as a service-oriented architecture. The concept of a service-oriented architecture allows a product to decide autonomously about the production process by using services of various devices, which are integrated into the production network. In the case of biological processes, the data obtained from the measurements of the cell culture such as pH or confluence is used to adapt the process by execution of different services or changing service parameters (Figure 2).
For this purpose, each laboratory device is represented as an autonomous virtual device in a production network. This device provides services to the network which can be used to execute different processes such as media change or measurement of pH or confluence. By establishing standardized communication interfaces and defined process rules, a high degree of system flexibility combined with a reliably PLC-driven device level can be achieved.
An example of where this concept has been successfully implemented is the StemCellFactory project, in which an automated platform for the cultivation and reprogramming of induced pluripotent stem cells (iPSCs) has been developed [11,12]. iPSCs are often used for drug testing and can be generated by reprogramming patient-specific cell material such as skin cells. However, growth behavior and, by extension, the time until a culture is confluent does not only differ between the cells from different donors but can also vary between the different clones after reprogramming. Therefore, each culture needs to be treated individually. Within the StemCellFactory, every cell culture is assigned to a separate protocol. Each culture can use the services provided by the laboratory devices according to the assigned workflow independently. Using the data obtained during regular measurements of the cell culture, the protocol can be adjusted via the execution of different services or changing the service parameter for an optimal cell treatment (Figure 3).
Metrics & data analytics
With advances in computerization, data tracking and networking that is characteristic of Industry 4.0, increasing amounts of information becomes available for automated data processing. The results that can be achieved however largely depend on the software, as data analysis is only as good as the implemented algorithm. Cell cultures often exhibit an inherent biological complexity that reflects in phenotypic morphology or in growth behavior. In contrast to processes performed by lab technicians, who can draw from years of training and experience, computerized systems often lack the capability of adapting to changes and atypical situations.
In recent years, machine learning has become a powerful technology that can potentially be used to tackle this challenge. For example, machine-based neuronal networks are used to detect traffic signs or written letters. The adaptation of this technology will potentially facilitate reproducible cell culture assessment and the characterization of phenotypes. Moreover, the implementation of machine learning concepts can not only be used to mimic the learning processes every technician undergoes when performing repeated tasks; with advances in the development of improved and novel sensors, more information will become available during the cultivation process. In combination with big data analysis, the generation of large amounts of data potentially can reveal correlations between different factors that have been previously undiscovered. Additionally, this allows for the implementation of a sophisticated feedback control of the culture where fully automated processes are employed.
Translational insight
In the past, biological manufacturing processes were mainly characterized by manual labor. Whilst appropriate for the production of biopharmaceuticals, for the large-scale generation of patient specific therapies, many challenges are lying ahead.
In order to provide a safe treatment option, cell products have to be well characterized and produced in a standardized manner. The implementation of automation technology promises to establish reproducible and robust processes. Moreover, implementation of Industry 4.0 concepts offers solutions for process understanding and control that go beyond. The generation and analysis of large data volumes will play a key role in establishing adaptive process layouts. In combination with machine learning, this will further drive process understanding and control.
While we have been mainly discussed the implementation of automated concepts for 2D cultures, these concepts promise to be even more powerful when transferred into bioreactor-based systems. Since bioreactors allow a higher degree of parameter control and already provide a high level of process analytical technology, a much greater level of process control can be achieved. Here, process automation and data analytics can support existing process design concepts based on statistic and methodic approaches such as Design of Experiment and Quality by Design. Ultimately, the combination of these tools and technologies promises to transform cell production standards in order to achieve safe and well defined products for cell and gene therapy in the future.
Financial disclosure
The authors are employees of the Fraunhofer Institute of Production Technology IPT in Aachen, Germany, and are funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement no 667932. Apart from this the authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Trounson A, Thakar RG, Lomax G et al. Clinical trials for stem cell therapies. BMC Med. 2011; 9, 52. doi: 10.1186/1741-7015-9-52
2. Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J. Stem Cells 2014; 6(2): 82–93. doi: 10.4252/wjsc.v6.i2.82
3. Murphy B. An Industry Perspective on the Clinical Preparation of Cell Therapies. Cell Gene Therapy Insights 2016; 2(2): 243–248. doi: 10.18609/cgti.2016.032
4. Rafiq QA, Hewitt CJ. Cell therapies: Why scale matters. Pharmaceutical Bioprocessing 2015; 3(2): 97–99. doi: 10.4155/pbp.14.63
5. Heathman TR, Rafiq QA, Chan AK et al. Characterization of human mesenchymal stem cells from multiple donors and the implications for large scale bioprocess development. Biochem. Eng. J. 2016; 108: 14–23. doi: 10.1016/j.bej.2015.06.018
6. Hourd P, Ginty P, Chandra A et al. Manufacturing models permitting roll out/scale out of clinically led autologous cell therapies: regulatory and scientific challenges for comparability. Cytotherapy 2014; 16(8): 1033–1047. doi: 10.1016/j.jcyt.2014.03.005
7. Alliance for Regenerative Medicine (ed) Pharma and Biotech Survey 2014
8. 2016 49th Hawaii International Conference on System Sciences (HICSS)
9. Schenk FW, Brill N, Marx U et al. High-speed microscopy of continuously moving cell culture vessels. Sci. Rep. 2016; 6: 34038. doi: 10.1038/srep34038
10. StemCellFactory II – Automated Production Platform for the Standardized Generation of Human iPS Cells and iPS Cell-derived Human Cell Products for Compound Screening. 2015; www.stemcellfactory.de/
11. Kulik M, Elanzew A, Haupt S et al. Novel platform for fully automated generation and expansion of highly standardized iPS cells. J. Biotechnol. 2016; 231: S33-S34. doi: 10.1016/j.jbiotec.2016.05.136
Affiliations
Michael Kulik<*,
Corresponding author
Fraunhofer Institute for Production Technology IPT, Steinbachstraße 17, 52074 Aachen, Germany;
Phone: +49 241 8904 -411
Email: michael.kulik@ipt.fraunhofer.de
Jelena Ochs*,
Fraunhofer Institute for Production Technology IPT, Steinbachstraße 17, 52074 Aachen, Germany;
Phone: +49 241 8904-571
Email: jelena.ochs@ipt.fraunhofer.de
Niels König
Fraunhofer Institute for Production Technology IPT, Steinbachstraße 17, 52074 Aachen, Germany;
Phone: +49 241 8904 -113
Email:niels.koenig@ipt.fraunhofer.de
Prof. Dr.-Ing. Robert Schmitt
Laboratory for Machine Tools and Production Engineering (WZL), RWTH Aachen University, Steinbachstr. 19, 52074 Aachen,
Fraunhofer Institute for Production Technology IPT, Steinbachstraße 17, 52074 Aachen, Germany;
Phone: +49 241 80 20283
Email: r.schmitt@wzl.rwth-aachen.de
*These authors contributed equally to this article