Recent advances in conditional cell immortalization technology
Cell Gene Therapy Insights 2016; 2(3), 339-355.
10.18609/cgti.2016.044
Gene-modified cell therapies are transforming medicine. Over the last 18 months, notable clinical successes using antigen-targeting cellular immunotherapies have been achieved. However, another kind of gene modification has significant potential for the cell therapy industry. The development of fully controllable transgenes has enabled the creation of conditionally immortalized cells that can be expanded to clinical quantities in a stable and consistent fashion, yet can be returned to a normal, non-dividing state for therapeutic delivery to the patient. In this article, we discuss some of the key technologies that have been used to create conditionally immortalized cells for clinical development.
The pressure and need to develop stable cell lines as biomedical research tools has triggered numerous approaches to produce stable, well-characterized cells. Big Pharma in particular relies on stable cell lines for drug screening and toxicology studies. Whilst embryonic stem cells and induced pluripotent stem cells invoke some powerful arguments for their utility, due to the large numbers of cells that can be generated and, in case of the latter, the ability to create patient- or disease-specific attributes to be studied, there are also some limitations with respect to consistent and reliable differentiation into target cells of interest. To this end, production of cell lines using primary cells from a more advanced stage of development than those of the early embryo, coupled with inducible transgene technologies that can impart regulatory control over cell division, offer an alternative strategy.
Conditional immortalization uses inducible transgene technology to create a cells that can be expanded in a consistent fashion when the transgene is active. If the transgene was permanently activated then the cells would, in theory, continuously divide. However, it is critical for conditional immortalization technology that the transgene is operator controllable so when desired clinical quantities of cell material are achieved, the transgene can be de-activated by the operator, returning the cells to a normal, post-mitotic state. This ensures that the cell formulation delivered to the patient is safe and carries negligible risk of cancer, overcoming the major concern for constitutively immortalized cells; cells with an infinite proliferative potential could become cancerous if they acquire oncogenic mutations.
Conditional immortalization involves inserting a modified gene that can be regulated by a defined reagent, controlled by the operator. In one set of conditions where the reagent is present, the transgene is active and the cells divide ceaselessly. In a second set of conditions where the reagent is removed, the cells are no longer immortal but have the potential to behave as would be expected under normal conditions such as undergoing differentiation (Figure 1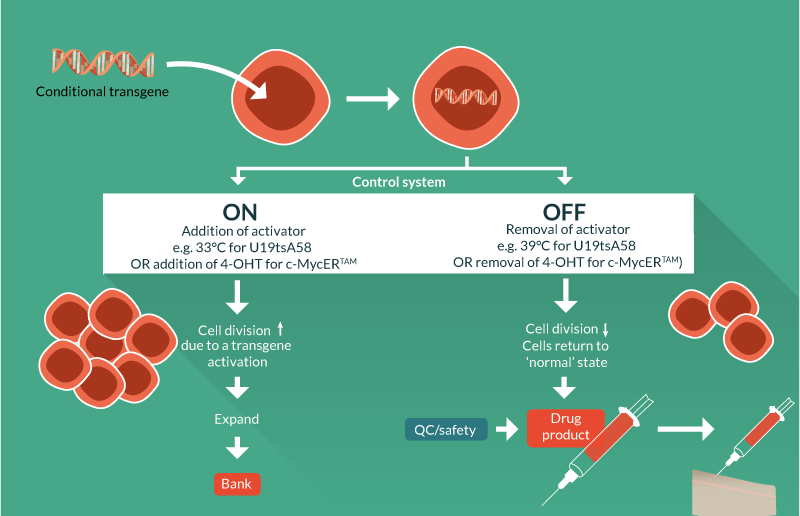
A natural progression from utility as biomedical research tools has been to develop conditionally immortalized cells as therapeutics. Although still in early development with few cell lines developed, some of these candidate therapies are already being utilized in pre-clinical and clinical studies with notable success. An overview of technologies, methods and associated patents is presented in Tables 1–3 & Figure 2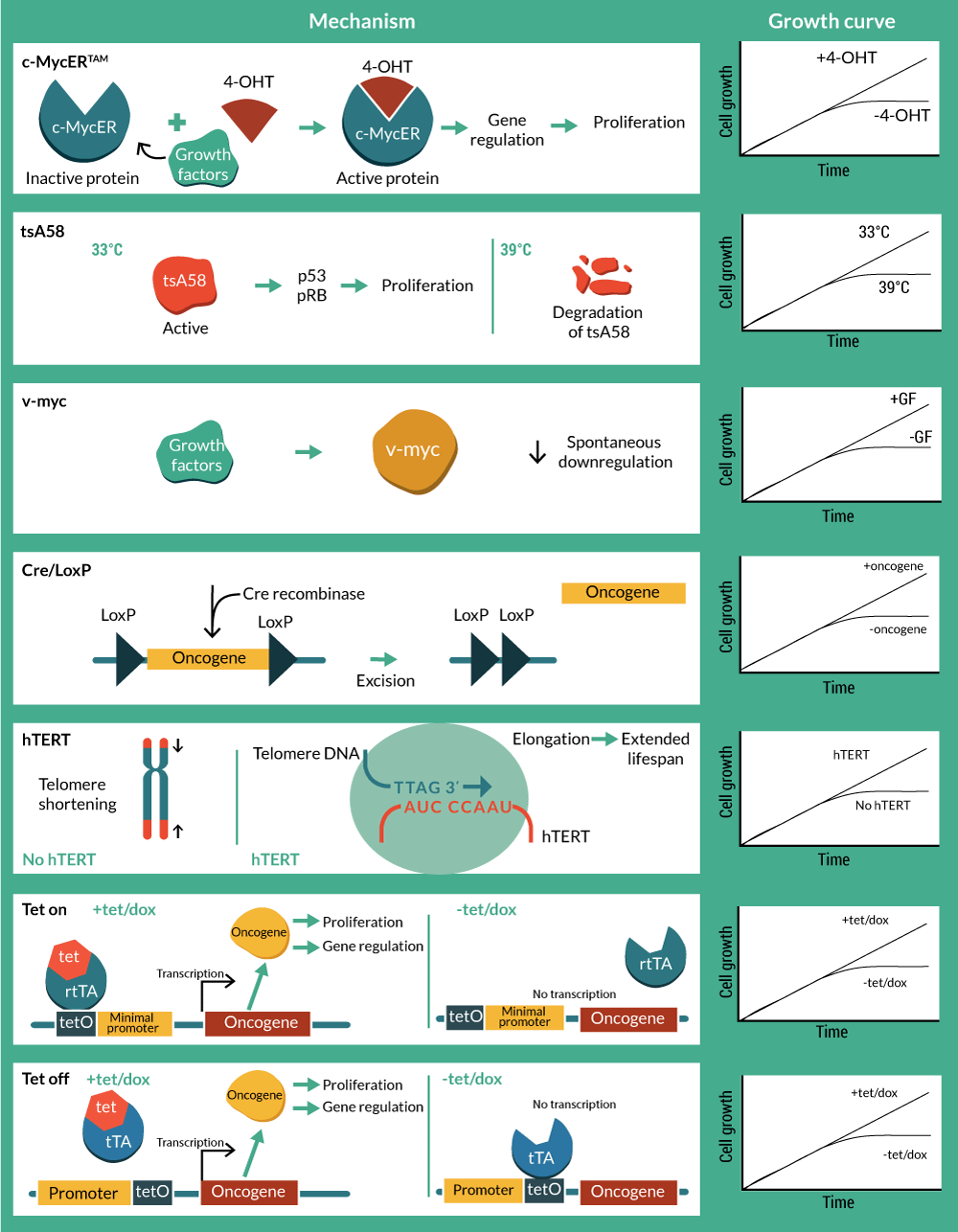
Immortalization is induced by multiple mechanisms
Normal human somatic cells undergo a finite number of cell divisions before they enter into a non-dividing state called senescence [3,4]. This natural process acts as an intrinsic anti-tumor mechanism. Some cells such as fibroblasts can undergo 50–60 population doublings before becoming senescent, whereas other cells such as breast luminal epithelial cells (the cell type from which most breast cancers are derived) only undergo a few divisions in culture. This natural process and the variation in replicative lifespan across different cell types limits the generation of new cell lines through simple culture of normal cells, particularly if the commercial agenda is to deliver industrial quantities of allogeneic cell product. In fact, early cell lines developed for research purposes were obtained simply from tumors that grew readily in culture [5,6].
It wasn’t until the advent of genetic engineering and procedures for the delivery of transgenes via DNA transfection or by viruses to insert desired immortalizing genes into the genome that scientists were able to create immortalized cells that were not derived from tumors. There is no single universal method to immortalize equally every candidate cell type for therapy. The type of cell and species from which it is obtained are factors that affect this. For example, many fundamental studies have been conducted in mice, but mouse cells have long telomeres so it is suggested that they do not undergo replicative senescence as human cells do, but stress-induced senescence. Even though stress activation of the p53 and pRB pathways are common causes, growing mouse cells in perfect conditions (e.g., optimal media and oxygen) would prevent the stress that leads to activation of these pathways. Perfect conditions are extremely challenging to define and so further genetic modifications are required. Human cells, in addition to silencing of p53 and pRB, also require telomere maintenance, for example via telomerase reconstitution. Therefore immortalizing cells is a complex process since not all cells can be immortalized using a single genetic tool. Moreover, simply immortalizing cells would generate large quantities of cells for drug screening but this cell material would not be suitable for implantation into patients due to the lack of cell cycle regulatory controls and the inability to turn off the genetic modification driving cell division.
Conditional immortalization
Genetic tools can be created that in addition to immortalizing cells, offer regulatory control via elements activated by external factors that switch on and off cell proliferation in a way that is controlled by the operator. This provides the basis for continuous cell culture with cell proliferation that can yield high cell numbers of consistent quality in a way that is scalable and cost-effective (Figure 3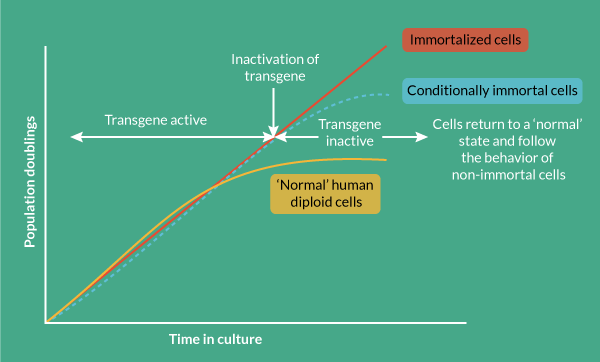
Myc activation
c-myc, along with the viral homolog v-myc, exerts regulatory control over a range of cell functions, but in particular it drives cell cycle entry and cell division. This makes it an attractive target for creating stable cell lines and in fact c-myc is one of the four Yamanaka factors used to create induced pluripotent stem cells [13]. Mutations in the myc gene that result in it being constitutively expressed are associated with oncogenic transformation, resulting in cancer. Therefore, controlled expression of c-myc is desired, and preferably under full operator control. The conditional immortalization technology c-MycERTAM consists of a fusion gene that encodes a chimeric protein, composed of c-myc and an N-terminal truncated hormone binding domain of a mutant murine estrogen receptor (G525R), which can no longer bind to 17β-estradiol and estrogen, but is responsive to activation by the presence of the synthetic estrogen-like agonist 4-hydroxytamoxifen (4-OHT) [14,15].
In contrast with its wild-type version, the hormone binding domain of the mutant G525R has 1,000-fold lower binding affinity to estradiol, but retains its affinity to 4-OHT due to an amino acid change from glycine to arginine in the position 525 [15]. This means that culturing cells in the presence of 4-OHT promotes c-myc activity and subsequent cell division, whereas in the absence of 4-OHT, the cells revert to a non-activated state and can undergo maturation as normal cells do.
Before the development of c-MycERTAM, the application of wild-type hormone receptors for fusion protein generation with viral antigens presented significant challenges. These included concern about inadequate control of activation due to presence of estrogens in serum and weak agonists in basal culture media. Moreover, circulating hormones could activate the c-MycERTAM in vivo and drive continued proliferation, posing a safety risk [14].
The synthesis of c-MycERTAM does not affect the phenotype of the cells, and this conditional immortalization technology has been used for the development of human stem cell lines from cortical neuroepithelium, which have been investigated in pre-clinical animal studies for ischemic stroke [11,16–19], limb ischemia [20] and are currently being investigated in clinical trials as a treatment for stroke disability (Phase 1 and 2) and in Phase 1 trial for clinical limb ischemia (Table 2). Results of Phase 1 for ischemic stroke revealed that intracerebral doses of CTX0E03 (from 2 to 20 million cells) in 11 male patients did not cause adverse effects, and therapy showed improvement in function [2]. Therefore, c-MycERTAM has significant clinical potential with clinical trials so far displaying favorable results. However, c-MycERTAM is just one of a number of molecular tools that can impart regulatory control over cell division for potentially generating cells for patients.
Among myc oncogenes, the avian viral homolog v-myc has also proven to effectively immortalize human neural stem cells (hNSCs) [21–24]. Similarly to its cellular counterpart, v-myc transduced hNSC growth and differentiation are dependent on mitogenic stimulation by growth factors. Spontaneous downregulation of the avian v-myc after 24–48 hours of engraftment in neonatal mice implied a safety precedent for clinical applications [22,25,26]. Established v-myc hNSC cell lines have shown potential as delivery vehicles for selective gene therapy due to their tumor-tropic properties [26,27]. Preclinical studies of a hNSC line (HB1.F3.CD) genetically modified to express cytosine deaminase, resulted in tumor site conversion of 5-fluorocytosine to the chemotherapeutic 5-fluorouracil [28]. Currently, a Phase 1 clinical trial is undergoing to study doses and side effects of this anti-cancer strategy (ID: 13401 NCI-2013-02346 13401).
Temperature-sensitive large tumor antigen of SV40
SV40 is a double-stranded DNA virus of rhesus monkey origin. SV40 has a number of antigens, including large tumor antigen (Tag), as well as several others [29,30]. However, it is the large Tag that is significant. Tag regulates cell signaling pathways that induce cells to enter into S phase and undergo a DNA damage response that facilitates viral DNA replication. Tag also binds to and inactivates the p53 and pRB family of proteins, powerful tumor suppressors involved in cell cycle progression and apoptosis, to create an ideal environment permissive for viral replication [8,29,31,32].
Early work with rodent cells showed that Tag immortalized these cells such that they acquired infinite proliferative potential [33]. Inactivation of Tag then subsequently resulted in rapid and irreversible loss of proliferative potential in G1 and G2 phases of the cell cycle, demonstrating that Tag is continuously required to maintain the proliferative state [9,34]. These traits made Tag an ideal candidate for developing controllable cell lines.
Inactivation of Tag was achieved using a temperature-sensitive mutant of the large Tag (SV40 tsA58) that had originally been isolated in 1975 [35] and found to behave as wild type at the permissive temperature (33.5°C), but biologically inactive at the non-permissive temperature of 39 ºC [35]. This mutant was selected for the development of a vector (pZipSVtsA58) that included the early region of tsA58 for the analysis of the Tag in the transformation of primary rat cells [9,36].
In contrast to rodent cells, overriding normal cell cycle checkpoints in human cells with Tag results in an extension of growth potential beyond normal senescence and cells then undergo ‘crisis’ where abortive or abnormal mitosis occurs and leads to cell death [37].
Telomerase
Much work has been carried out to define the underlying pathways that regulate the finite lifespan of normal cells and how these are overcome when cells become immortal, particularly in cancer research [38]. The process is likely to involve two components, a mitotic ‘clock’ that counts the number of divisions and entry into a post-mitotic state.
In human somatic cells, the progressive shortening of telomeres, short repetitive sequences at the ends of chromosomes, with each cell division has been proposed to be the mitotic clock [39]. Human telomeres comprise multiple tandem repeats of TTTAGG located at chromosome ends. They are dependent on the enzyme telomerase to maintain their length, but as human somatic cells do not express telomerase at levels sufficient to maintain the telomeres, they shorten by around 50 base pairs at each cell division [10,40]. Collectively, telomere loss in conjunction with the lack of telomerase activity is the mitotic clock responsible for limiting the number of divisions before senescence [3,7].
Although it was originally proposed that reconstitution of telomerase activity using hTERT was sufficient for immortalization of primary human cells [3], others found that reconstitution of telomerase alone could not, and in these instances secondary inactivation of regulator pathways such as p16 and pRB was required [8,41,42]. In addition, the studies assessed constitutive telomerase activation. However, telomerase has, in combination with other conditional transgenes, proven very successful in supporting conditional immortality.
Temperature-sensitive SV40 versus telomerase
Experiments by O’Hare et al. validated earlier observations that hTERT alone was insufficient to immortalize freshly isolated human mammary fibroblast and endothelial cells [10]. The U19 mutant is defective for binding SV40 origin of replication [43] and when delivered in a recombinant retrovirus encoding a U19 Tag, was more efficient at immortalizing rodent cells than wild-type Tag [33]. As a consequence of this work, a vector incorporating both tsA58 and U19 mutations was constructed to create a murine oligodendrocyte precursor cell line capable of in vitro differentiation [44].
The O’Hare et al. study showed that ectopic expression of hTERT or U19tsA58 Tag alone was not sufficient for immortalization of freshly isolated human cells but a combination of the genes resulted in efficient generation of immortal cells lines irrespective of the order in which they were introduced. However, the order and timing of introducing the two genes did influence the genetic stability of the cells, which is a significant consideration for generating safe cells for therapy. They further showed that maintenance of immortalization depended on a continued expression of functional U19tsA58 Tag, with hTERT alone unable to maintain growth when the U19tsA58 Tag was inactivated [10].
U19tsA58 Tag was also capable of creating a conditional immortal cell line from rat neonatal optic nerve there was capable of differentiating into oligodendrocytes [45]. The same group also used U19tsA58 Tag to study the heterogeneity of candidate regenerative olfactory ensheathing cells from olfactory bulb and lamina propria [46]. These studies in rat using cells that are difficult to culture and characterize offer promise for human cell line generation for regenerative purposes.
Combining hTERT & U19tsA58 Tag
The demonstration that hTERT and U19tsA58 Tag synergized to efficiently immortalize human cells led to the development of a bicistronic retrovirus, which simultaneously expresses hTERT, U19tsA58 Tag and an antibiotic resistance gene. This virus is highly efficient for immortalization of human cells. From this work, US Patent 6399384 B1 was assigned to ReNeuron Ltd and the Ludwig Institute for Cancer Research (Table 3).
Although this vector is highly efficient at immortalizing human cells, the resulting cells had a higher number of chromosomes than normal, even though both genes had been transduced simultaneously. The likely reason for this karyotypic instability was unmasked using a yeast 2-hybrid screen and rodent studies. It was found that Tag interacts with the spindle assembly checkpoint protein, Bub 1, and this makes Tag containing cells ‘leaky’ to this checkpoint, which results in the two daughter cells acquiring an unequal number of chromosomes [47]. Furthermore, the interaction of Tag with Bub1 is not required for immortalization but closely correlates with transformation. Further work showed that Tag binding to Bub1 breaches genome integrity leading to a DNA damage response, p53 stabilization and tetraploidy [30].
A potential solution to this problem of karyotypic instability would be to construct an SV40 triple mutant that in addition to the U19tsA58 Tag double mutant, lacks the Bub1 interaction site (U19dl89-97tsA58). A very simple way to facilitate making cell lines using such a mutant would be to develop a bicistronic vector, as described in US Patent 6399384 B1.
Cre-loxP system for reversible immortalization
The potential safety concerns with temperature sensitive genetic tools for clinical application, led to the consideration of site-specific recombination systems to excise the oncogene [48–50]. The Bacteriophage p1 Cre is an enzyme that promotes recombination in specific sites called loxP. When two 33 bp loxP sequences are oriented, recombination occurs and consequently the intervening sequence is cleaved and removed [49,51]. The application of reversible immortalization by Cre-loxP is promising for both autologous and allogeneic cell therapy. Biopsies and primary cultures can be immortalized with a recombinant oncogene flanked with loxP sites. Efficient transfection with Cre will result in the excision of the immortalizing genes. After oncogene removal, cells should be identical to the primary culture population but in increased numbers [50,52]. Cre-loxP system has been applied from rat adrenal cells to human hepatocytes and myogenic cells with hTERT and Tag as immortalizing genes [48,50,53–58]. The cre-lox system is not 100% efficient and therefore there is a requirement to eliminate cells that have not deleted the transgene. Negative controls for recombination have included a Herpes simplex virus 1-thymidine kinase (HSV-TK) suicide gene in order to kill the small portion of refractory immortalized cells in the presence of ganciclovir (GCV) after Cre transfection [53,59,60]. However, as the system requires an exhaustive selection process, tamoxifen-dependent Cre recombinases have been incorporated in order to achieve a better-controlled excision of the oncogene [54,58,60,61].
Tet-On & Tet-Off: transcription regulation of immortalization
Conditional immortalization has also been achieved by the use of transcription-regulated systems. The most widely used have been derived using the prokaryotic tetracycline repression system. They utilize a tet repressor (tetR) protein, that binds strongly to a sequence called the tetracycline operator (tetO) in the absence of the antibiotic (tetracycline or doxycycline). When the antibiotic is present, it binds to the repressor, thereby inhibiting it’s binding to the tetO. The first system available was called ‘Tet-Off’, and was developed in HeLa cells [62]. In this system the tet repressor binding site is inserted between the promoter and the transcriptional start site such that binding of the repressor sterically blocks transcription. However the steric hindrance is readily overcome upon addition of small amounts of tetracycline and doxycycline that prevent binding of the tetR to the tetO, thereby inducing reporter gene expression. However, Tet-Off systems require constant doses of tetracycline to activate transcription and also easily lose tet regulation due to loss of the tetR. To circumvent this, ‘Tet-On’ systems were generated [63]. Gossen and Bujard fused the tetR with the C-terminal activation domain of the virion protein 16 (VP16) from herpes simplex virus (HSV) to generate a hybrid transactivator (tTA) that stimulates promoters fused to tetO sequences. A modification of four amino acids resulted in a reverse tetracycline transactivator (rtTA), which binds to tetO only in the presence of tetracycline or doxycycline. Oncogenes (c-Myc and Tag) and telomerase (hTERT) were initially tested in Tet-based immortalization systems for mouse embryo fibroblasts (MEFs), murine kidney cells (293T), mouse embryonic stem cells and human endothelial cells [64–67].
More recently, mesenchymal stromal cells (MSCs) have been immortalized with tetracycline inducible systems. Tetracycline-inducible hTERT-expressing MSC cell lines were created by Piper et al. [68]. These cell lines retained multipotency and immortalization was dependent on telomere elongation. However, additional screening was necessary to determine which clones showed the lowest levels of hTERT basal expression. Leakiness is the most criticized drawback of tetracycline-based expression systems.
A conditionally immortalized MSC line was generated by lentiviral transfection of Tag-hTERT in conjunction with a doxycycline/tetracycline-induction (Tet-On) system [69]. These cells were used to study senescence-associated DNA methylation (SA-DNAm) changes, and could be maintained in culture for 80 days without any sign of senescence. Removal of doxycycline in the media resulted in immediate growth arrest, and further expression of senescence-associated ß-galactosidase. Telomere length increased significantly when the cells were exposed to the antibiotic and were not affected with SA-DNAm.
Conclusion
The emerging cell and gene therapy industry will grow stronger by having access to new and powerful molecular tools that enable the creation of conditionally immortal therapeutic cell lines from adult cells that have potential curative or regenerative effects in their natural state, but that cannot be expanded to consistently high yields in this natural state. Immortalization on its own carries concerns associated with genetic instability and transformation to a cancer phenotype. The conditional step overcomes this by utilizing a fully controllable mechanism that removes or permanently silences the immortalization gene prior to delivery.
There are a range of different molecular biology tools that can be used to create conditionally immortalized cells that are operator controllable, via manipulation of reagents and environment, which offer potential solutions to the industrial scale generation of cells for patients.
Clearly there is commercial value in the creation of conditionally immortalized cell lines for therapeutic application. There are challenges to be addressed for other technologies, such as ensuring that the transgene is completely silenced prior to delivering cells to the patient. The lessons from early prominent successes like ReNeuron’s will hopefully unlock development opportunities more widely across the industry through increased understanding of how to generate the necessary safety data, navigate regulatory pathways and create commercially sustainable manufacturing processes that are cost effective and have a sound reimbursement model.
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Tables
| Commonly used conditional immortalization technologies. | |||
|---|---|---|---|
| Insert | Description | IP related | Ref. |
| c-MycERTAM | A fusion protein comprising a growth promoting gene, c-Myc, and a hormone receptor that is regulated by a synthetic drug, 4-OHT. Cells are expanded with complete media plus 4-OHT, cell differentiation is triggered by removal of growth factors and 4-OHT from the growth media | Patent CA2521421A1, DE602005002430D1,DE602005002430T2, EP1645626A1,EP1645626B1, US7416888, US7419827,US766667, US20060067918,US20060104959, US20080118479 Patent US7544511, US20090263901, US 20020064873 A1 Patent US 20090238799 A1, CA2628408A1, EP1957523A1, WO2007052036A1 | [14] |
| Temperature-sensitive simian virus SV40 T antigen | A thermolabile large T antigen encoded by the simian virus 40 early-region mutant tsA58. Expansion is carried out at the permissive temperature of 33°C or at the nonpermissive temperature of 37°C to facilitate cell differentiation | Patent US5866759 A Patent US 6399384 B1, CA2383253A1, CN1373804A,DE60020855D1, DE60020855T2,EP1212420A1, EP1212420B1,WO2001021790A1 Patent US20070274969, CA2536655A1, EP1664098A1, US20090252715, WO2005026201A1 | [35,36,44] |
| v-myc | p110gag-myc protein encoded in the avian myelocytomatosis virus genome. v-myc is spontaneously downregulated after differentiation | Patent US7186409, US20020045261, US20070031391, US 7655224 B2 Patent US 20020115213 A1, US20050169897, US20080152590 | [70,71] |
| E6/E7 | Oncoproteins from human papilloma virus type 16 (HPV16) E6 and E7 cooperate in mediating-cellular immortalization. They inactivate tumor suppressors such as p53 and pRB. | Patent US 5376542 A, US5576206, WO1993021958A1 Patent CA2324479A1, CA2324479C, CN1299409A, DE69919531D1, DE69919531T2, EP1071747A2, EP1071747B1, US20020042133, WO1999054435A2, WO1999054435A3 | [83,12,84,85] |
| hTERT | Catalytic unit of human telomerase reverse transcriptase. The enzyme catalyzes the synthesis of 6-bp repeats to elongate telomeres As basal levels of telomerase in primary human cells are not enough for an unlimited lifespan. Transduction of exogenous hTERT can result in the extension of lifespan. In some cases the cooperation of hTERT with an oncogene is required | Patent US 6399384 B1, CA2383253A1, CN1373804A,DE60020855D1, DE60020855T2,EP1212420A1, EP1212420B1,WO2001021790A1 | [42,86,10] |
References
1. Muir KW. Pilot Investigation of Stem Cells in Stroke Phase II Efficacy (PISCES-II) 2014. Website
2. Kalladka D, Sinden J, Pollock K et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet 2016; 388(10046), 787–96. CrossRef
3. Bodnar AG, Ouellette M, Frolkis M et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279(1998), 349–52. CrossRef
4. Hayflick L, Moorhead PS. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961; 1. CrossRef
5. Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. J. Exp. Med. 1953; 97(5), 695–710. CrossRef
6. Noble M, Groves a K, Ataliotis P, Ikram Z, Jat PS. The H-2KbtsA58 transgenic mouse: a new tool for the rapid generation of novel cell lines. Transgenic Res. 1995; 4, 215–25. CrossRef
7. Maqsood MI, Matin MM, Bahrami AR, Ghasroldasht MM. Immortality of cell lines: Challenges and advantages of establishment. Cell Biol. Int. 2013; 37(2005), 1038–45. Website
8. Kiyono T, Foster S, Koop JI, McDougall JK, Galloway D, Klingelhutz J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 1998; 396, 84–8. CrossRef
9. Jat PS, Sharp P. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol. Cell Biol. 1989; 9(4), 1672–81. CrossRef
10. O’Hare MJ, Bond J, Clarke C et al. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc. Natl Acad. Sci. USA 2001; 98(2), 646–51. CrossRef
11. Pollock K, Stroemer P, Patel S et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 2006; 199(1), 143–55. CrossRef
12. Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 1989; 63(10), 4417–21. Website
13. Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006; 126(4), 663–76. CrossRef
14. Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995; 23(27), 1686–90. CrossRef
15. Danielian PS, White R, Hoare SA, Fawell SE, Parker MG. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol. Endocrinol. 1993; 7, 232–40. CrossRef
16. Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. Neurogenesis after experimental stroke in a dose-dependent fashion. Neuorehabil. Neural Repair. 2009; 23(9), 895–909. CrossRef
17. Smith EJ, Stroemer RP, Gorenkova N et al. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells 2012; 30(4), 785–96. CrossRef
18. Pollock K, Sinden JD. Progressing Neural Stem Cell Lines to the Clinic Introduction : Clinical Indications for Transplant Therapy. In: Shi Y, Clegg DO (editors). Stem Cell Research and Therapeutics. 2008; 105–22. CrossRef
19. Hicks C, Stevanato L, Stroemer RP, Tang E, Richardson S, Sinden JD. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2012; 22, 1541–52. CrossRef
20. Katare R, Stroemer P, Hicks C et al. Clinical-grade human neural stem cells promote reparative neovascularization in mouse models of hindlimb ischemia. Arterioscler. Thromb. Vasc. Biol. 2014; 34, 408–18. CrossRef
21. Villa A, Snyder EY, Vescovi A, Martínez-Serrano A. Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from the human CNS. Exp. Neurol. 2000; 161(1), 67–84. CrossRef
22. Flax JD, Aurora S, Yang C et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat. Biotechnol. 1998; 16(11), 1033–9. CrossRef
23. Cho T, Bae JH, Choi HB et al. Human neural stem cells: electrophysiological properties of voltage-gated ion channels. Neuroreport 2002; 13(11), 1447–52. CrossRef
24. Kim SK. PEX-Producing Human Neural Stem Cells Inhibit Tumor Growth in a Mouse Glioma Model. Clin. Cancer Res. 2005; 11(16), 5965–70. CrossRef
25. Snyder EY. Neural stem-like cells: Developmental lessons with therapeutic potential. Neuroscientist 1998; 4(6), 408–25. CrossRef
26. Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008; 15(10), 739–52. CrossRef
27. Frank RT, Edmiston M, Kendall SE et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS One 2009; 4(12), 2–8. CrossRef
28. Aboody KS, Najbauer J, Metz MZ et al. Neural Stem Cell-Mediated Enzyme/Prodrug Therapy for Glioma: Preclinical Studies. Sci. Transl. Med. 2013; 5(184), 184ra59–184ra59.
29. Martini F, Corallini A, Balatti V, Sabbioni S, Pancaldi C, Tognon M. Simian virus 40 in humans. Infect. Agent Cancer 2007; 2, 13. CrossRef
30. Hein J, Boichuk S, Wu J et al. Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding. J. Virol. 2009; 83(1), 117–27. CrossRef
31. Lock RL, Benvenuti S, Jat PS. Immortalization by Sv40 Large T Antigen. In: Stein GS, Pardee AB (editors). Cell Cycle and Growth Control: Biomolecular Regulation and Cancer. Second. 2004; 15–92. CrossRef
32. An P, Sáenz Robles MT, Pipas JM. Large T antigens of polyomaviruses: amazing molecular machines. Annu. Rev. Microbiol. 2012; 66, 213–36. CrossRef
33. Jat P, Sharp P. Large T-Antigens of Simian Virus-40 and Polyomavirus Efficiently Establish Primary Fibroblasts. J. Virol. 1986; 59(3), 746–50. Website
34. Gonos ES, Burns JS, Mazars GR et al. Rat embryo fibroblasts immortalized with simian virus 40 large T antigen undergo senescence upon its inactivation. Mol. Cell. Biol. 1996; 16(9), 5127–38. CrossRef
35. Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J. Virol. 1975; 15(3), 613–8. Website
36. Frederiksen K, Jat PS, Valtz N, Levy D, McKay R. Immortalization of precursor cells from the mammalian CNS. Neuron 1988; 1, 439–48. CrossRef
37. Shay JW, Wright WE, Werbin H. Defining the molecular mechanisms of human cell immortalization. Biochim. Biophys. Acta 1991; 1072(1), 1–7. CrossRef
38. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144(5), 646–74. CrossRef
39. Allsopp RC, Vaziri H, Patterson C et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl Acad. Sci. USA 1992; 89(0027–8424 ), 10114–8. Website
40. Harley CB, Futcher a B, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345, 458–60. CrossRef
41. Hahn WC, Hahn WC, Counter CM et al. Creation of human tumour cells with defined genetic elements. Nature 1999; 400, 464–8. CrossRef
42. Counter CM, Hahn WC, Wei W et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl Acad. Sci. USA 1998; 95(25), 14723–8. CrossRef
43. Paucha E, Kalderon D, Harvey RW, Smith AE. Simian virus 40 origin DNA-binding domain on large T antigen. J. Virol. 1986; 57(1), 50–64. Website
44. Almazan G, McKay R. An oligodendrocyte precursor cell line from rat optic nerve. Brain Res. 1992; 579, 234–45. CrossRef
45. Barnett SC, Franklin RJ, Blakemore WF. In vitro and in vivo analysis of a rat bipotential O-2A progenitor cell line containing the temperature-sensitive mutant gene of the SV40 large T antigen. Eur. J. Neurosci. 1993; 5, 1247–60. CrossRef
46. Franceschini IA, Barnett SC. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev. Biol. 1996; 173(27), 327–43. CrossRef
47. Cotsiki M, Lock RL, Cheng Y et al. Simian virus 40 large T antigen targets the spindle assembly checkpoint protein Bub1. Proc. Natl Acad. Sci. USA 2004; 101(4), 947–52. CrossRef
48. Westerman KA, Leboulch P. Reversible immortalization of human hepatocytes mediated by retroviral transfer and site-specific recombination. Proc. Natl Acad. Sci. USA 1996; 93, 8971–6. CrossRef
49. Paillard F. Reversible cell immortalization with the Cre-lox system [comment]. Hum. Gene Ther. 1999; 10(10), 1597–8. CrossRef
50. Berghella L, De AL, Coletta M et al. Reversible immortalization of human myogenic cells by site-specific excision of a retrovirally transferred oncogene. Hum. Gene Ther. 1999; 10(10), 1607–17. CrossRef
51. Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl Acad. Sci. USA 1988; 85(14), 5166–70. CrossRef
52. Cascio SM. Novel strategies for immortalization of human hepatocytes. Artif. Organs. 2001; 25(7), 529–38. CrossRef
53. Kobayashi N. Prevention of Acute Liver Failure in Rats with Reversibly Immortalized Human Hepatocytes. Science 2000; 287(5456), 1258–62. CrossRef
54. Zhao L, Li J, Lv G et al. Evaluation of a reversibly immortalized human hepatocyte line in bioartificial liver in pigs. African J. Biotechnol. 2012; 11(17), 4116–26.
55. Eaton MJ, Herman JP, Jullien N, Lopez TL, Martinez M, Huang J. Immortalized chromaffin cells disimmortalized with Cre/lox site-directed recombination for use in cell therapy for pain after partial nerve injury. Exp. Neurol. 2002; 175(1), 49–60. CrossRef
56. Duplan H, Li RY, Vue C et al. Grafts of immortalized chromaffin cells bio-engineered to improve met-enkephalin release also reduce formalin-evoked c-fos expression in rat spinal cord. Neurosci. Lett. 2004; 370(1), 1–6. CrossRef
57. Scharfmann R, Pechberty S. Development of a conditionally immortalized human pancreatic β cell line. J. Clin. Invest. 2014; 124(5), 1–12. CrossRef
58. Wu HL, Wang Y, Zhang P et al. Reversible immortalization of rat pancreatic ß cells with a novel immortalizing and tamoxifen-mediated self-recombination tricistronic vector. J. Biotechnol. 2011; 151(3), 231–41. CrossRef
59. Nguyen TH, Mai G, Villiger P et al. Treatment of acetaminophen-induced acute liver failure in the mouse with conditionally immortalized human hepatocytes. J. Hepatol. 2005; 43(6), 1031–7. CrossRef
60. Totsugawa T, Yong C, Rivas-Carrillo JD et al. Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with Tamoxifen-mediated self-recombination. J. Hepatol. 2007; 47(1), 74–82. CrossRef
61. Zhang Y, Riesterer C, Ayrall AM, Sablitzky F, Littlewood TD, Reth M. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 1996; 24(4), 543–8. CrossRef
62. Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. USA 1992; 89(12), 5547–51. CrossRef
63. Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science 1995; 268(5218), 1766–9. CrossRef
64. May T, Butueva M, Bantner S et al. Synthetic gene regulation circuits for control of cell expansion. Tissue Eng. Part A 2010; 16(2), 441–52. CrossRef
65. May T, Hauser H, Wirth D. Transcriptional control of SV40 T-antigen expression allows a complete reversion of immortalization. Nucleic Acids Res. 2004; 32(18), 5529–38. CrossRef
66. May T, Wirth D, Hauser H, Mueller PP. Transcriptionally regulated immortalization overcomes side effects of temperature-sensitive SV40 large T antigen. Biochem. Biophys. Res. Commun. 2005; 327(3), 734–41. CrossRef
67. Anastassiadis K, Rostovskaya M, Lubitz S, Weidlich S, Stewart AF. Precise conditional immortalization of mouse cells using tetracycline-regulated SV40 large T-antigen. Genesis 2010; 48(4), 220–32. CrossRef
68. Piper SL, Wang M, Yamamoto A et al. Inducible immortality in hTERT-human mesenchymal stem cells. J. Orthop. Res. 2012; 30(12), 1879–85. CrossRef
69. Koch MC, Reck K, Shao K et al. Pluripotent stem cells escape from senescence-associated DNA methylation changes. Genome Res. 2013; 23: 248–59. CrossRef
70. Land H, Parada LF, Weinberg R. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 1983; 53(9), 1689–99. CrossRef
71.Ryder EF, Snyder EY, Cepko CL. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J. Neurobiol. 1990; 21, 356–75. CrossRef
72. ReNeuron Ltd. Pilot Investigation of Stem Cells in Stroke (PISCES) [Internet]. 2010. Website
73. ReNeuron Ltd. Safety Trial Of CTX Cells In Patients With Lower Limb Ischaemia. 2013. Website
74. ReNeuron Ltd. Observational Study of Ischaemic Stroke (OSIS). 2013.
75. Portnow J. Genetically Modified Neural Stem Cells, Flucytosine, and Leucovorin for Treating Patients With Recurrent High-Grade Gliomas. Website
76. Portnow J. A Pilot Feasibility Study of Oral 5-Fluorocytosine and Genetically-Modified Neural Stem Cells Expressing E.Coli Cytosine Deaminase for Treatment of Recurrent High Grade Gliomas. 2010. Website
77. Hasan SM, Vugler AA, Miljan EA, Sinden JD, Moss SE, Greenwood J. Immortalized human fetal retinal cells retain progenitor characteristics and represent a potential source for the treatment of retinal degenerative disease. Cell Transplant. 2010; 19(10), 1291–306. CrossRef
78. McGinley LM. Human Cortical Neural Stem Cells Expressing Insulin- Like Growth Factor-I: A Novel Cellular Therapy for Alzheimer’s Disease. Stem Cells Transl Med. 2016; 5, 379–91. CrossRef
79. Miljan E, Hines SJ, Pande P et al. Implantation of c-mycERTAM immortalized human mesencephalic-derived clonal cell lines ameliorates behavior dysfunction in a rat model of Parkinson’s disease. Stem Cells Dev. 2009; 18(2):307–19. CrossRef
80. Amemori T, Romanyuk N, Jendelova P et al. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res. Ther. 2013; 4(3), 68. CrossRef
81. Cocks G, Romanyuk N, Amemori T et al. Conditionally immortalized stem cell lines from human spinal cord retain regional identity and generate functional V2a interneurons and motorneurons. Stem Cell Res. Ther. 2013; 4(3), 69. CrossRef
82. Villa A, Liste I, Courtois ET, Seiz EG, et al. Generation and properties of a new human ventral mesencephalic neural stem cell line. Exp. Cell Res. 2009; 315(11), 1860–74. CrossRef
83. Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989; 8(12), 3905–10.
84. Romanczuk H, Villa LL, Schlegel R, Howley PM. The viral transcriptional regulatory region upstream of the E6 and E7 genes is a major determinant of the differential immortalization activities of human papillomavirus types 16 and 18. J. Virol. 1991; 65(5), 2739–44.
85. Schlegel R, Phelps WC, Zhang YL, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988 ; 7(10), 3181–7.
86. Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 1997; 90(4), 785–95.
Affiliations
Ivan B Wall1§, Gerardo Santiago Toledo1 & Parmjit Jat2
1Department of Biochemical Engineering, UCL, London, UK
2Department of Neurodegenerative Disease/MRC Prion Unit, UCL Institute of Neurology, London, UK
§Author for correspondence
wall@ucl.ac.uk

This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.