Successful ex vivo expansion of pro-angiogenic cells from one single primitive HSC
Cell Gene Therapy Insights 2016; 2(2), 199-217.
10.18609/cgti.2016.019
Objective: Bone marrow cell therapy has pioneered the field of regenerative medicine for the treatment of ischemic disease, but remains a controversial topic due to persistent uncertainty on purity and quality of the cell product. Here, we evaluated the therapeutic activity of pro-angiogenic clones derived from single-sorted long-term repopulating hematopoietic stem cells (LT-HSCs, CD150+/CD34–/Lineage-/Sca-1+/c-Kit+) in a murine model of hindlimb ischemia. Approach and Results: C57BL/6 mice underwent unilateral limb ischemia by femoral artery occlusion, followed by intramuscular injection of green fluorescent BM LT-HSCs cells (2,000 per mouse) from congenic donor C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ mice or vehicle (PBS). Cell therapy with LT-HSCs markedly accelerated the recovery of blood flow to the ischemic limb as measured by laser Doppler flowmetry (P2, P<0.05) and arteriole density (108 ±6 vs. 72 ±9/mm2, P<0.05). Additionally, LT-HSC therapy improved the viability and proliferation of resident vascular cells. In separate experiments, single sorted BM LT-HSC-derived cells were expanded up to 40,000 fold in culture giving rise to two distinct clone subsets, according to the expression level of the surface marker CD31. Upon transplantation in the mouse ischemic model, CD31high LT-HSC-derived cells showed superior pro-angiogenic and pro-healing activities as compared with CD31low LT-HSC-derived cells. Conclusions: These data establish clonogenic CD31high LT-HSC-derived cells as a promising cell therapy approach for the treatment of limb ischemia.
Critical limb ischemia (CLI), the end stage of peripheral artery disease (PAD), is caused by severe obstruction of blood flow resulting in unbearable pain, ulcers, and high risk for amputation [1]. Surgical bypass or percutaneous revascularization, the gold standard for the treatment of CLI, exert only temporary symptomatic alleviation. Furthermore, around one third of patients with CLI cannot be revascularized because of multivascular disease or occlusions of small-caliber blood vessels [2,3]. Gene and cell therapies have been therefore proposed as a possible complement or alternative to interventional angioplasty [4]. Clinical trials using bone marrow (BM)-derived cells showed significant therapeutic benefit, including improvement of ankle brachial index, transcutaneous partial pressure of oxygen, pain relief, and decreased risk of amputation [5]. However, despite initial evidence of safety and efficacy, BM cell therapy remains a controversial topic due to the lack of unambiguously defined isolation protocols, delineation of differentiation hierarchy and quality/quantity standards. Furthermore, the seminal concept of BM cells directly participating in de novo post-natal vasculogenesis, proposed some years ago by Asahara et al., have been revised in light of the novel indication that paracrine mechanisms, rather than the conversion of one cell type to another, are responsible for reciprocal interactions between hematopoietic and vascular cells within the BM microenvironment as well as after transplantation in peripheral tissues [6–9]. In line, BM vascular cells are able to recreate the hematopoietic microenvironment following transplantation at heterotopic sites, through the release of angiopoietin-1 and activation of the Notch signaling pathway [10,11]. Reciprocally, hematopoietic cells promote neovascularization of peripheral tissues principally through the release of soluble and packaged angiogenic factors [12,13].
Initial evidence suggests that pro-angiogenic cells (PACs) are particularly enriched in the fraction of multipotent long-term repopulating hematopoietic stem cells (LT-HSCs), which in the mouse are identified as CD34–/c-Kit+/Sca-1+/Lineage– (CD34–/KSL) cells [14]. However, CD34–/KSL cells represent a heterogeneous population containing sub-fractions with diverse regenerative capacity. Further characterization by use of the Signaling Lymphocyte Activation Molecule (SLAM) marker CD150 allows identification of a fraction of CD34–/KSL cells endowed with high self-renewal potential and repopulating capacity [14,15]. It remains unknown however if the same approach may be useful to establish a pro-angiogenic hierarchy within the population of LT-HSCs.
We propose that the CD150 marker may help select proper PACs from the bulk population of CD34–/KSL cells and that transplantation of small dosage CD150+/CD34–/KSL cells may benefit the healing of ischemic limbs. Results confirm the presence of clonogenic PACs in the mouse BM CD150+/CD34–/KSL cell fraction. Additionally, we discovered that CD31high-expressing clones derived from single sorted CD150+/CD34–/KSL cells possess superior and more durable pro-angiogenic and pro-survival activities in vivo. Our refined approach provides new impetus to BM cell therapy for the treatment of CLI.
Materials & Methods
Ethics
Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (the Institute of Laboratory Animal Resources, 1996) and with approval of the British Home Office and the University of Bristol.
Isolation of CD150+/CD34–/KSL HSCs
Bone marrow was extracted from 10–12-week-old male C57BL/6 (Harlan, UK) mouse tibia and femur. A total of six mice were used per isolation. Total BM cells were washed with ice-cold Hank’s balanced salt solution containing 0.5% bovine serum albumin and 0.02% sodium-azide. Cells were resuspended and stained in this buffer with anti-Lineage mixture (Biotin, Miltenyi Biotec, Germany) anti Sca-1 (PE, eBioscience, CA USA), anti c-Kit (APC-eFluor 780, eBioscience), anti-CD34 (Alexa-Fluor 700, eBioscience) and anti CD150 (APC, eBioscience). Streptavidin-PE-Cy7 conjugated antibodies were used as secondary for anti-Lineage mixture.
Flow cytometry analysis
To identity surface markers on KSL-selected and clonogenically expanded cells, fluorescence activated cell sorting (FACS) analysis was performed. Surface expression of mouse Flk-1, CD31, Tie-2 and c-Kit were determined using a PE-conjugated anti Flk-1 (BD Biosciences, CA, USA), BV421-conjugated anti CD31 (BD), APC-conjugated anti Tie-2 and APC-eFluor 780-conjugated anti c-Kit (eBioscience). Flow cytometry was performed on a FACSCalibur (BD) equipped with FACSDiva software (BD).
Hindlimb ischemia model & cell transplantation
Male 10–12 week-old C57BL/6J mice (Harlan) underwent unilateral hindlimb ischemia by ligature of the proximal end of the femoral artery. Immediately after ischemia induction, 2,000 GFP+, CD150+/CD34–/KSL cells from congenic mice or vehicle (PBS, 30 μL) were injected into three different points of the ischemic adductor muscle (n = 6 animals per group). A second set of experiments were performed comparing clonogenically expanded CD150+/CD34–/KSL cells, according to levels of CD31 expression: three groups were studied, with mice randomized to receive CD31high/CD150+/CD34–/KSL or CD31low/CD150+/CD34–/KSL cells or vehicle (n = 5 animals per group). Blood flow recovery was followed up by laser Doppler flowmetry at day 3, 7 and 21. The Doppler data were normalized as the mean ratio of ischemic to contralateral limb measurements.
Source of labelled BM-HSCs for cell tracking after in vivo transplantation
Donor BM-HSCs were obtained as described above from male 10–12-week-old C57BL/6J (Harlan) GFP congenic mice (C57BL/6-Tg (CAG-EGFP) 10sb/J) (The Jackson Laboratory, ME, USA).
Evaluation of capillary & arteriolar density
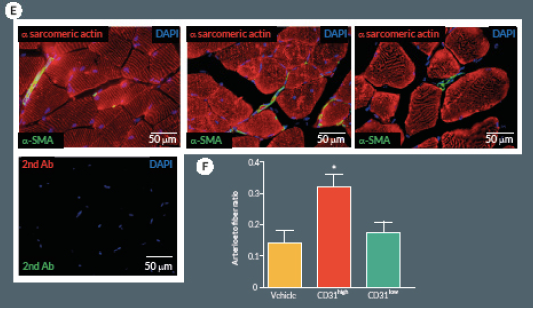
At day 21, mice were perfused under physiologic pressure with paraformaldehyde 4% PBS. Adductor muscles were isolated and embedded in OCT. Capillary and arteriolar densities were assessed with the use of isolectin B4 (Invitrogen, UK) and α-smooth muscle actin (α-SMA; Sigma) staining as reported [16]. Briefly, histological sections of mouse adductor muscles were incubated for 1 hour at room temperature (RT) with biotin-conjugated isolectin B4 followed by Alexa-488 streptavidin-conjugated antibody (Invitrogen) to identify endothelial cells and Cy3-conjugated α-SMA to identify smooth muscle cells. Incubation with α-sarcomerin actin (Sigma) followed by Alexa-568 secondary antibody (Invitrogen) was performed in order to identify myofibers. Slides were observed under a fluorescence microscope (Olympus CX41, Olympus, UK). Arterioles were distinguished from the capillaries and veins by the structure of tunica media in the vessel wall and the shape of the lumen. Isolectin-positive capillaries and α-SMA-positive arterioles were counted within 10 random fields per section (400x) (n = 5–9 animals per group). Results were expressed as capillary or arteriole to fiber ratio.
Assessment of apoptosis & proliferation
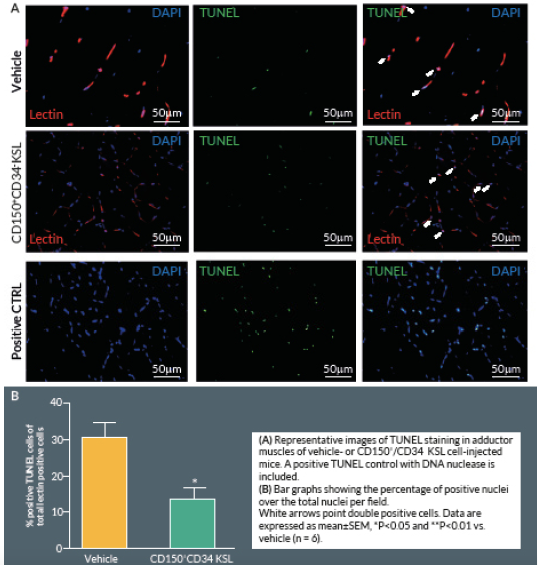
At Day 21, mice were perfused under physiologic pressure with paraformaldehyde 4% PBS. Adductor muscles were isolated and embedded in OCT. Histological cryosections were labelled by TUNEL in order to identify apoptotic cells. Briefly, 4% formaldehyde-fixed cryosections were pre-treated with proteinase K (1:50) for 15 min at RT. The TUNEL assay was performed using a TdT-Fluor in situ apoptosis detection kit (Trevigen, MD, USA). Sections were counterstained with DAPI to identify nuclei. Ten random fields from each section were captured (400x) for counting apoptotic cells (n = 5–9 animals per group). A counterstaining with biotin-conjugated isolectin B4 followed by Alexa-568 streptavidin-conjugated antibody (Invitrogen) was performed. TUNEL-positive cells with bright green nuclei were counted and expressed as numbers of TUNEL-positive nuclei over the total nuclei of positive lectin cells in the captured field. A treatment with TACS™ Nuclease (Trevigen) has been carried out as positive control.
Proliferating cells were identified by Ki67 staining. Briefly, 4% formaldehyde-fixed cryosections were incubated overnight at 4° C with rabbit anti-mouse Ki-67 antibody (Abcam, UK), followed by Alexa-488-conjugated anti-rabbit secondary antibody (Invitrogen) for 2 hours at room temperature. Counterstaining with biotin-conjugated isolectin B4 was followed by Alexa-568 streptavidin-conjugated antibody. Ten random fields were captured (400x) (n = 5–9 animals per group). Ki-67 positive cells with bright green nuclei were counted and expressed as numbers of Ki-67 positive nuclei over the total nuclei of positive lectin cells in the captured field.
Ex vivo expansion of single sorted CD150+/CD34–/KSL HSCs
A colony forming assay (CFA) was performed as previously described [17,18]. Single cells of mouse primitive LT-HSCs (CD150+/CD34–/KSL) were sorted into each well of a 96-well culture plate (Greiner bio-one, UK) using a Becton Dickinson Influx high speed cell sorter (BD). The sorted cells were cultured for 2 weeks in methylcellulose medium M3236 (Stem Cell Technologies, Canada) supplemented with 20 ng/ml Stem Cell Factor (SCF; R&D Systems, UK), 50 ng/mL VEGF (R&D Systems, UK), 20 ng/ml interleukin-3 (IL3; PeproTech, NJ, USA), 50 ng/mL Epidermal Growth Factor (EGF; PeproTech), 50 ng/mL Insulin-like Growth Factor-1 (IGF-1; PeproTech), and 2 U/mL heparin (Sigma).
Real time (RT)-PCR to detect gene expression in cultured cells
RNA was extracted using the Trizol method (Thermo Fisher) using glycogen as a RNA carrier (Thermo Fisher). The extracted RNA was purified by acid phenol-chloroform phase separation and re-precipitated using isopropanol. The cDNA library was generated using 100 ng of isolated RNA, which underwent reverse-transcription by the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher). Quantitative real-time PCR was performed using specific DNA primers (Sigma-Aldrich) and the Power Sybr reaction mix (Thermo Fisher) on the Light Cycler 480 II system (Roche Diagnostics, Switzerland) (Table 1). Expression was normalized to that of the housekeeping genes GAPDH.
| Table 1. Sequence of gene-specific primers used for qRT-PCR. | |
|---|---|
| Primer | Sequence |
| Ang1 fwd | AAC CTC ACC CTG CAAAGATG |
| Ang1 rvs | CAC AGA TGG CCT TGA TGT TG |
| Ang2 fwd | CAAGGC ACTGAG AGACAC |
| Ang2 rvs | TGC GCT TCA GTC TGG TAC AC |
| VEGF fwd | TCTCCC AGATCG GTG ACAGT |
| VEGF rvs | GGG CAG AGC TGA GTG TTA GC |
| SCF fwd | AAC TATGTC GCC GGG ATG GA |
| SCF rvs | CTTCCATGC ATA ACA CGA GGTCA |
| SDF-1 fwd | CTG TAG CCTGAC GGACCAAT |
| SDF-1 rvs | CCA TTC TAC AGG AGG CCA AA |
| Tie2 fwd | TAC AAC GGC CAT TTC TCC TC |
| Tie2 rvs | GTG GCT TGC TTG GTA CAG GT |
| CXCR4 fwd | GCC AAG TTC AAAAGC TCTGC |
| CXCR4 rvs | AGC CTC TGC TCA TGG AGT TG |
| Thrombospondin1 fwd | GAA GTA CCC ACC TGC TTC CA |
| Thrombospondin1 rvs | TTT GGA GAG TGG ACC CAAAG |
| JAG1 fwd | AGA GAC AGG CAG GCG ATCT |
| JAG1 rvs | TGG GAT GCT TCC AAC TTC A |
| Dll1 fwd | CGG GAA AAA GAA AAC GTG TG |
| Dll1 rvs | ATA GAC CCG AAG TGC CTT TGT |
| Dll3 fwd | CTG GCC AGC CTA GAA CTC TG |
| Dll3 rvs | GAG TGG TGC ACG CCT TTA AT |
| Dll4 fwd | AGG CAA GAG TTG GTC CTT CC |
| Dll4 rvs | TTT AGC ATG AAG GCC CTG AG |
| Notch1 fwd | ATA GCA TGA TGG GGC CAC TA |
| Notch1 rvs | GGC AGG CCC TGG TAA ATA AT |
| Notch2 fwd | TTC CAG CTT ATC CCA AAA CG |
| Notch2 rvs | GGC ATC TCT GGG ATC TGG TA |
| Notch3 fwd | CTA GCC CAG CAA CTG CTA CC |
| Notch3 rvs | GGA ACA GAT ATG GGG TGT GG |
| Notch4 fwd | AAA TAA CCG TTA AGC TCA CTT GTC T |
| Notch4 rvs | GGT AGG CGA CAC TCG GTT CCC |
| GAPDH fwd | TGT GAA CGG ATT TGG CCG TA |
| GAPDH rvs | ACT GTG CCG TTG AAT TTG CC |
Statistical analysis
All results are represented as mean ± SEM. Differences between multiple groups were compared by analysis of variance (ANOVA) followed by a Holm-Sidak multiple comparison test. Two-group analysis was performed by t-test (unpaired as appropriate).
Results
Frequency of primitive HSCs
We first used flow cytometry to verify the relative abundance of primitive HSCs in murine BM. The frequency of CD150+/CD34–/KSL cells among total BM cells was around 0.002% ± 0.0002 (Figure 1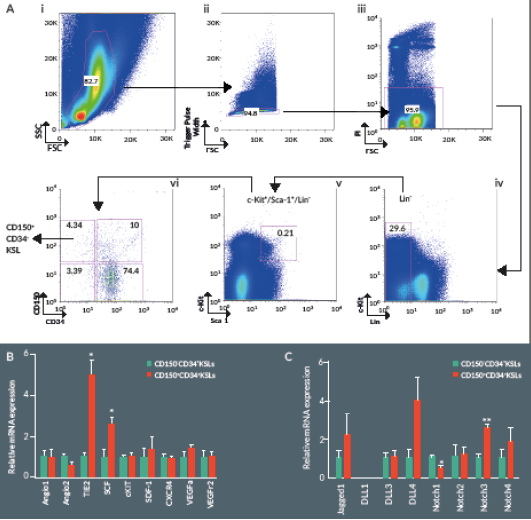
Expressional profile of CD150+/CD34–/KSL cells
Stem cell factor (SCF, also known as c-Kit ligand) in concert with other cytokines and growth factors contributes to the self-renewal and maintenance of HSCs in the BM niche. Analysis of gene expression by RT-PCR indicates higher expression levels of SCF and the angiopoietin receptor Tie2 in CD150+/CD34–/KSL cells as compared to CD150–/CD34–/KSL cells (Figure 1B). In addition, the Notch signaling pathway represents a key mechanism in the regulation of HSC self-renewal and differentiation. Expressional analysis indicates higher levels of Notch3 and lower levels of Notch1 in the CD150+ fraction of CD34–/KSL cells (Figure 1C).
Low dosage CD150+/CD34–/KSL cell therapy improves hemodynamic recovery & reparative angiogenesis in a mouse model of hindlimb ischemia
Preclinical cell therapy trials using total or fractioned BM cells showed the requirement of large dosages, generally exceeding 1 x 106, to successfully improve hemodynamic recovery in mice with operative limb ischemia [16,19]. Importantly, an amount of 5×103 of KSL cells was able to achieve a similar improvement in functional neovascularization, thus suggesting that this cell population is greatly enriched for therapeutic angiogenic properties when compared to total BM cells [20]. In order to further define the truly salutary fraction within the bulk BM cell population, we performed a cell therapy study using a very low dosage of CD150+CD34– KSL cells. Results indicate that intra-muscular injection of 2 x 103 cells produces a significant acceleration in blood flow recovery as compared with vehicle (P < 0.05, Figure 2 A & B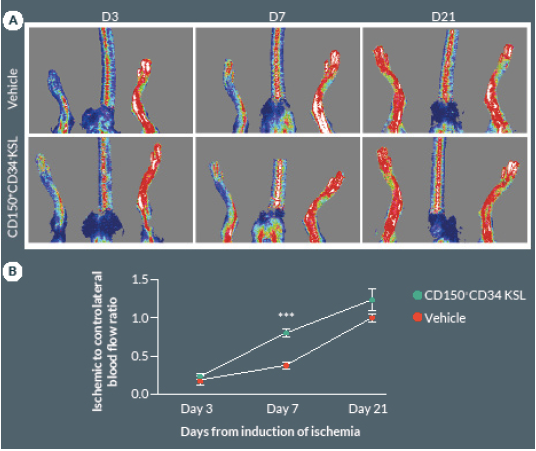
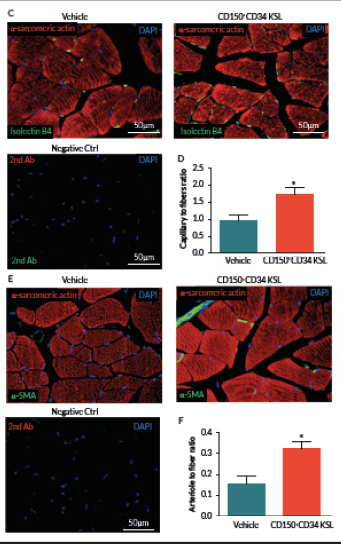
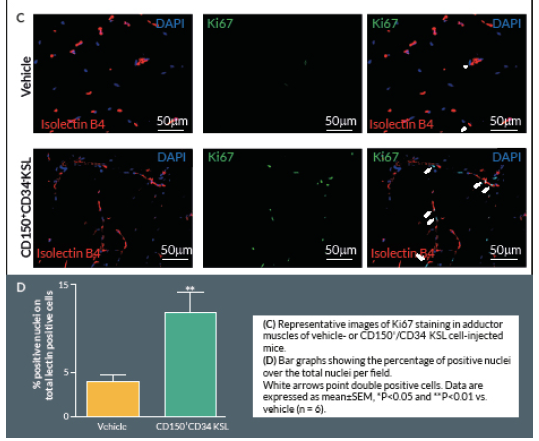
Clonogenic expansion of single CD150+CD34– KSL cells
Having demonstrated that CD150+CD34– KSL cell therapy is effective at a very low dosage, we next focused on the possibility of further restricting cell heterogeneity and eventually capturing the cell clone(s) responsible for the improvement of post-ischemic outcomes. To this end, we performed a single cell sorting of the CD150+CD34– KSL population, followed by clonogenic expansion. Single-sorted cells were cultured for 2 weeks in methylcellulose medium M3236 supplemented with growth factors, such as SCF, VEGF, IL3, EGF, IGF-1, as previously described by Tanaka et al [17]. Under these conditions, sorted cells from three independent experiments formed colonies with a frequency of 8 ± 1%, with an average colony size of 22,083 ± 1,007 cells in 2 weeks (Figure 4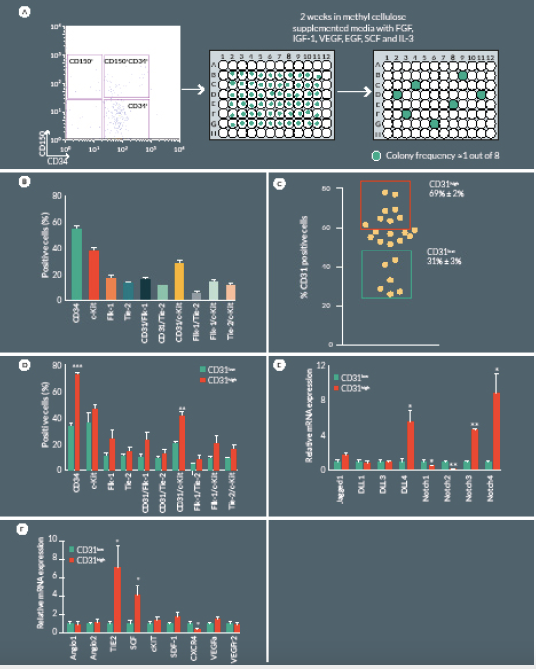
Successfully cultured CD150+CD34– KSL cells were then characterized by flow cytometry to assess endothelial and progenitor markers. Clonogenic cells were positive for CD31 (54 ± 3%), c-Kit (37 ± 2%) and to a lower extent for Flk1 (16 ± 2%) and Tie2 (12 ± 1%) (Figure 4B). Previous investigations indicate that CD31 is a unique marker of a full spectrum of non-endothelial hematopoietic BM cells that are tightly associated with neovascularization [19,21]. In our study, CD31 was variably expressed among different colonies emerging from single sorted CD150+CD34– KSL cells. In order to characterize specific features associated with the relative abundance of CD31 antigen, we performed additional expressional and in vivo studies comparing CD31high and CD31low colonies (Figure 4C). Flow cytometry indicate CD31high colonies tend to have higher expression levels of c-Kit, Flk-1 and Tie-2 although these difference did not statistical significance (Figure 4D), Restricting comparative analyses to the CD31+ sub-fraction within CD31high and CD31low colonies, we found that c-Kit is 2-fold more abundant in the former (P < 0.01 vs CD31low) (Figure 4D). Additional diversities were found at mRNA level with regard to the Notch signaling pathway, being DLL4 and Notch3 upregulated and Notch 2 downregulated in CD31high as compared with CD31low colonies (Figure 4E). Furthermore, the CD31high population showed an upregulated expression of SCF and Tie2, whereas CXCR4 was downregulated (Figure 4F).
CD31high cells improve hemodynamic recovery & reparative angiogenesis
Next we compared CD31high and CD31low cells vs. vehicle in the hindlimb ischemia model. We repeated the single cell sorting of the CD150+CD34– KSL population obtaining four different clones (Supplemental Figure 1A). We selected the highest and the lowest CD31 expressing clones (99.3 and 19.8% respectively) containing enough cells to perform cell therapy in biological replicates. Flow cytometry analysis of antigen co-expression indicates the relative enrichment of Flk-1 and c-Kit in the CD31high clone (Supplemental Figure 1B-D). Results of in vivo experiments are illustrated in Figure 5. Two-way ANOVA with repeated measurements identified an effect of time (P < 0.001) and cell treatment (P < 0.01) on blood flow recovery. Post-hoc analysis indicates that transplantation of CD31high cells derived from clonogenic CD150+CD34– KSL cells is able to induce a complete hemodynamic recovery at day 21. On the other hand, CD31low cells exerted only a transitory improvement in blood flow recovery at day 7, but failed to provide any additional beneficial effect over vehicle at day 21 (Figure 5A & B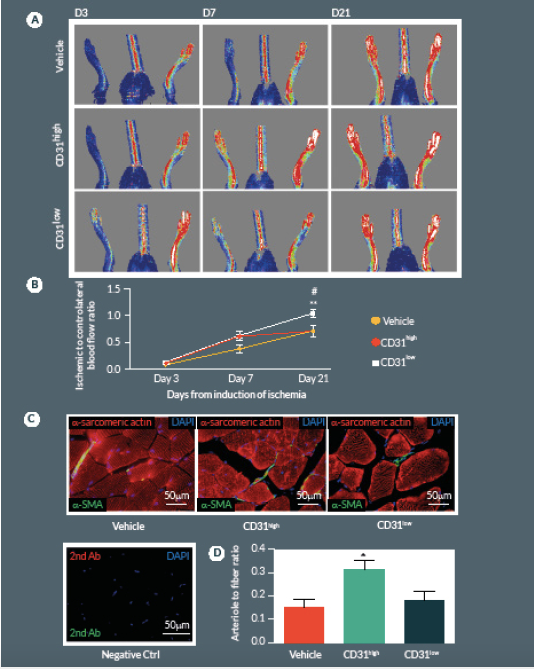
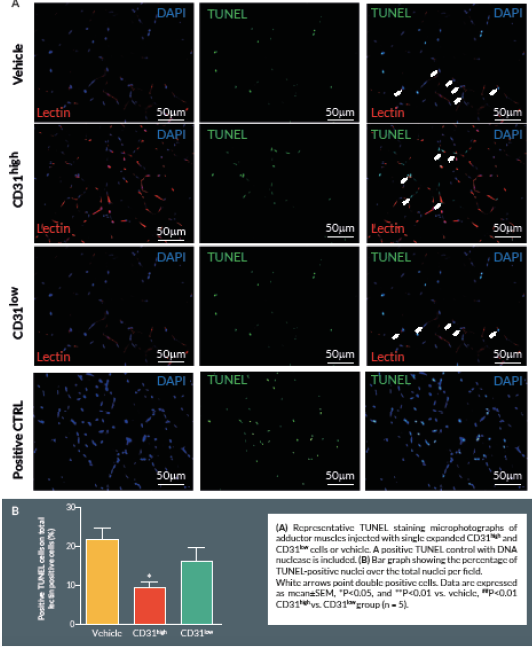
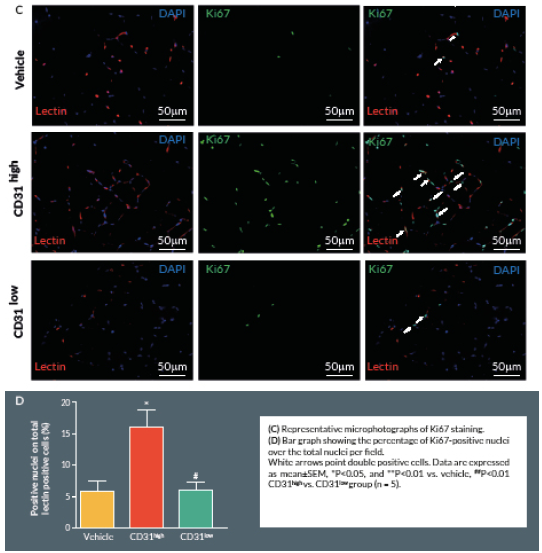
Discussion
This study demonstrates that a SLAM marker previously used to define the hierarchy of murine HSCs helps to distinguish truly pro-angiogenic elements within the total BM cell population. Furthermore, within CD150+CD34– KSL cells, we discovered that clonogenic cells expressing high levels of the CD31 antigen constitute the prevalent active component for stimulation of reparative angiogenesis in vivo.
Transplantation of BM-derived cells, including mononuclear cells, bona fide endothelial progenitor cells, mesenchymal stem cells, and HSCs were reported to promote therapeutic neovascularization in ischemic tissues. However, there are controversies in the field. In particular, unselected BM cells may comprise active as well as neutral and even counter-productive components, which may expose the recipient to adverse events including calcification, vascular plaque growth, and worsening of tissue ischemia [22–24]. To make matters even more complicated, patients carrying multiple cardiovascular risk factors, i.e., the subjects who would most benefit from cell therapy, possess fewer and dysfunctional PACs. Hence, methodological improvements are still required to achieve optimization of PAC manufacture. Two main approaches have been used so far to isolate PACs from BM cells:
- Culture and colony assays; and
- Selection based on surface markers [reviewed in 25,26].
However, both methods have been criticized for not being an ideal source of quality-assured cell products. Hybrid approaches based on two-step separation by immunomagnetic sorting for stemness markers followed by chemokine-directed migration have been proposed to obtain antigenically-defined sub-fractions with intact functional qualities [27].
Here we report an additional attempt to identify PACs from the primitive population of LT-HSCs. The first important outcome of this investigation is that transplantation of CD150+CD34– KSL cells provides robust stimulation of reparative angiogenesis in a mouse model of limb ischemia. The therapeutic effect is seemingly mediated by paracrine actions of injected cells as they could not be detected in the recipient muscle by use of a fluorescent marker. The second major result consists of the novel identification of clonogenic CD150+CD34– KSL cells expressing abundant levels of CD31 as the active component responsible for therapeutic effects in the hindlimb ischemia model. CD31, also known as platelet endothelial cell adhesion molecule 1, is a 130-kDa cell surface molecule and a member of the immunoglobulin superfamily. It reportedly plays important roles in leukocyte migration, cell–cell adhesion, and anti-apoptotic signalling [28,29]. It is noteworthy that recent studies have designated CD31 as a novel marker to identify murine and human PACs for the treatment of ischemic cardiovascular disease [19,21]. The above investigations indicate that CD31 is almost universally expressed among different BM cells and that both CD31+Lin+ or CD31+Lin- cells perform equally well in improving the recovery of ischemic limbs, suggesting that both mature and immature BM cells may be instrumental to tissue healing [19,21]. Our investigation applies the use of the CD31 marker to clonogenic cells derived from multipotent LT-HSCs, thereby allowing purification of the definitive cell product within the primitive fraction of BM cells. In agreement with previous studies, CD31 was expressed by multi-potent LT-HSCs [19,21]. Unexpectedly however, we found a wide variability in the abundance of this surface antigen among different clones. Most importantly, in vivo studies show a clear association between the abundance of CD31 and therapeutic activity, with CD31high/CD150+CD34– KSL cells exerting more complete and prolonged recovery from ischemia as compared with CD31low/CD150+CD34– KSL cells. Importantly, transplantation of clonogenic CD31high/CD150+CD34– KSL cells also resulted in salutary effects on viability and proliferation of resident vascular cells.
The Notch signalling pathway regulates multiple aspects of hematopoiesis. Ex vivo exposure of mouse or human HSCs to Notch ligands was shown to enhance cytokine-mediated expansion, leading to preclinical testing and early clinical trials using Notch-expanded progenitors to boost hematopoietic recovery [30]. However, whether Notch regulates PAC expansion and function remains unknown. Our preliminary data on gene expression identify a differential pattern in Notch components between the two clonogenic populations expressing high or low CD31 levels. Intriguingly, DDL4 was upregulated in CD31high/CD150+CD34– KSL cells. We have previously reported that an intact DDL4 signalling is essential for proper angiogenesis and recruitment of circulating cells along nascent vessels [31]. Likewise, we found CD31high/CD150+CD34– KSL cells express higher levels of Tie2, an angiopoietin receptor implicated in the development of definitive hematopoiesis and angiogenesis [32]. Further studies are necessary to understand the relevance of these expressional difference in relation to therapeutic outcomes of CD31high and CD31low cells.
In conclusion, the present study highlights the benefit of a purified population of primitive HSCs for the treatment of limb ischemia. Translation of the approach to human BMs could lead to the significant enhancement of current cell therapies for cardiovascular disease.
References
1. Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N. Engl. J Med. 2001; 344, 1608–21. CrossRef
2. Faglia E, Clerici G, Losa S et al. Limb revascularization feasibility in diabetic patients with critical limb ischemia: results from a cohort of 344 consecutive unselected diabetic patients evaluated in 2009. Diabetes Res. Clin. Pract. 2012; 95, 364–71.CrossRef
3. Spinetti G, Specchia C, Fortunato O et al. Migratory activity of circulating mononuclear cells is associated with cardiovascular mortality in type 2 diabetic patients with critical limb ischemia. Diabetes Care 2014; 37, 1410–7.CrossRef
4. Tongers J, Roncalli JG and Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation 2008; 118, 9–16.CrossRef
5. Matoba S, Tatsumi T, Murohara T et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am. Heart J. 2008; 156, 1010–8.CrossRef
6. Takahashi T, Kalka C, Masuda H et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999; 5, 434–8. CrossRef
7. Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014; 507, 323–8. CrossRef
8. Ding BS, Nolan DJ, Butler JM et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010; 468, 310–5. CrossRef
9. Ellis SL, Grassinger J, Jones A et al. The relationship between bone, hemopoietic stem cells, and vasculature. Blood 2011; 118, 1516–24. CrossRef
10. Sacchetti B, Funari A, Michienzi S et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007; 131, 324–36. CrossRef
11. Corselli M, Chin CJ, Parekh C et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood 2013; 121, 2891–901. CrossRef
12. Sahoo S, Klychko E, Thorne T et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011; 109, 724–8. CrossRef
13. Kumar AH, Metharom P, Schmeckpeper J, Weiss S, Martin K, Caplice NM. Bone marrow-derived CX3CR1 progenitors contribute to neointimal smooth muscle cells via fractalkine CX3CR1 interaction. FASEB J. 2010; 24, 81–92. CrossRef
14. Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med. 2010; 207, 1173–82. CrossRef
15. Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121, 1109–21. CrossRef
16. Sugihara S, Yamamoto Y, Matsuura T et al. Age-related BM-MNC dysfunction hampers neovascularization. Mech. Ageing Dev. 2007; 128, 511–6. CrossRef
17. Tanaka R, Wada M, Kwon SM et al. The effects of flap ischemia on normal and diabetic progenitor cell function. Plast. Reconstr. Surg. 2008; 121, 1929–42. CrossRef
18. Kwon SM, Eguchi M, Wada M et al. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation 2008; 118, 157–65. CrossRef
19. Kim H, Cho HJ, Kim SW et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ. Res. 2010; 107, 602–14. CrossRef
20. Kwon SM, Lee YK, Yokoyama A et al. Differential activity of bone marrow hematopoietic stem cell subpopulations for EPC development and ischemic neovascularization. J. Mol. Cell. Cardiol. 2011; 51, 308–17. CrossRef
21. Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J. Am. Coll. Cardiol. 2010; 56, 593–607. CrossRef
22. Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation 2004; 109, 3154–7. CrossRef
23. George J, Afek A, Abashidze A et al. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2005; 25, 2636–41. CrossRef
24. Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ. Res. 2004; 94, 230–8. CrossRef
25. Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J. Cell. Mol. Med. 2009; 13, 87–102. CrossRef
http://dx.doi.org/10.1111/j.1582-4934.2008.00598.x
26. Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012; 110, 624–37. CrossRef
27. Ascione R, Rowlinson J, Avolio E et al. Migration towards SDF-1 selects angiogenin-expressing bone marrow monocytes endowed with cardiac reparative activity in patients with previous myocardial infarction. J. Stem Cell Res. Ther. 2015; 6, 53. CrossRef
http://dx.doi.org/10.1186/s13287-015-0028-y
28. Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood 2004; 104, 1010–6. CrossRef
29. Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2007; 27, 2514–23. CrossRef
30. Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat. Med. 2010; 16, 232–6. CrossRef
31. Al Haj Zen A, Oikawa A, Bazan-Peregrino M, Meloni M, Emanueli C, Madeddu P. Inhibition of delta-like-4-mediated signaling impairs reparative angiogenesis after ischemia. Circ. Res. 2010; 107, 283-93. CrossRef
32. Takakura N, Huang XL, Naruse T et al. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 1998; 9, 677–86. CrossRef
ACKNOWLEDGEMENTS
We are thankful to the Flow Cytometry facility at the University of Bristol and to Dir. Andrew Herman. This study was funded by BHF program grant “Unravelling mechanisms of stem cell depletion for preservation of regenerative fitness in patients with diabetes”.
financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
ETHICAL CONDUCT OF RESEARCH
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
AFFILIATIONS
Corresponding Author:
Paolo Madeddu MD
Chair Experimental Cardiovascular Medicine
Bristol Heart Institute, University of Bristol, Level 7, Bristol Royal Infirmary, Upper Maudlin Street, Bristol, BS28HW, UKTel/Fax: +44 (0)1179 283904
madeddu@yahoo.com
