Japan’s regulatory framework: seeking to provide impetus to the commercialization of regenerative medicine products
Cell Gene Therapy Insights 2015; 1(1), 83-92
10.18609/cgti.2015.008
REGULATORY INSIGHT
The time is right for innovation in the fields of regenerative medicine and cell therapy in Japan. Beginning with the enactment of the Promotion Act on Regenerative Medicines in 2013, the regulatory framework has undergone sweeping reforms, including the establishment of the Act on the Safety of Regenerative Medicines (or RMS Act) and the Pharmaceutical and Medical Device Act (or PMD Act, formerly the Pharmaceutical Affairs Act) in November 2014. In this article, industry trends and the social impact of regenerative medicine and cell therapies will be discussed in the context of these recent legal and regulatory framework changes.
Current regulatory conditions
While Japan has achieved a certain presence in the area of foundational technology development, it lags behind in the areas of clinical research and development [1]. For instance, in the field of human embryonic stem cell (hESC) lines, excessive regulation has become entrenched, and Japan is now too far behind to catch up [2]. Several reports have been published on recent legal and regulatory changes regarding regenerative medicine and cell therapies, detailing the new framework [3-5]. Hara et al [4] provide an overview of the new regulatory framework for stem cell-based therapies in Japan prior to its settlement, in which they described procedures to provide regenerative medicine therapies under the Regenerative Medicines Act (RMS) according to a risk-based classification, a new approval process for regenerative medicine products under the PMD Act, and a new application scheme for regenerative medicine products for conditional time-limited and normal authorisation. Kokomi et al [3] provide a precise classification of the stem cell types used in regenerative medicine therapies, in which induced pluripotent stem cells (iPSCs), ESCs, cells in which a gene is introduced, xenogeneic cells and all cell types for allogeneic use are categorized as high risk (class I); somatic stem cells for autologous use are categorized as medium risk (class II); and somatic cells for autologous use are categorized as low risk (class III). Azuma [5] provides a detailed overview of the regulatory guidelines and descriptions which compiles key regulations and guidance documents under the PMD Act, regulations related to the RMS Act, a planned review scheme to provide regenerative medicine therapies, and other related legislation, including the Regenerative Medicine Promotion Act, the establishment of the Japan Agency for Medical Research and Development (AMED) [6], patent term extensions under the Patent Act, and the national health insurance reimbursement scheme.
Figure 1 presents an overview of the legal and regulatory changes relevant to regenerative medicine and cell therapies. In terms of their significance for the industry, the first point is that the field of regenerative medicine, which was previously vaguely defined, is now defined under law and has become subject to regulation. As a result of the change, two categories have been recognized namely regenerative medicine therapies under the RMS Act and regenerative medicine products under the PMD Act, and implementation frameworks have been established, in the case of the former primarily for medical institutions, and of the latter for corporations. The second point is the acceptance of external procurement from contracted manufacturing organizations for regenerative medicine therapies. As a result, regenerative medicine therapies are now expected to see industrial production and supply with practical involvement of corporations. The third point is the acceptance of an expedited approval system of regenerative medicine products. Specifically, under the new legislation, products need only be safety assured to be brought to market, paving the way for early use of new products. Furthermore, their efficacy can now be confirmed under conditions of actual use.
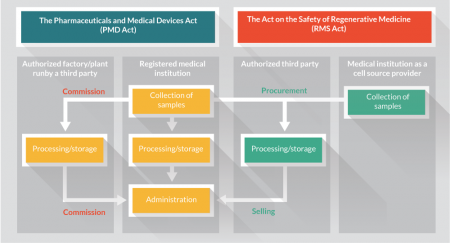
Figure 1. The new legal and regulatory framework in Japan. Schematic representation of schemes for regenerative medicine therapies and products [16]. All regenerative medicine therapies are executed under the Act on the Safety of Regenerative Medicine (RMS Act) where each registered medical institution holds an option for commissioned cell processing and storage by an authorized third party. All regenerative medicine products are provided under the Pharmaceutical and Medical Device Act (PMD Act) by authorised third party players which procure source cells from the medical institution or others and sell the processed/stored regenerative medicine products in a similar way to conventional pharmaceutical and medical products.
In addition, there a few important related policies. The first is a cabinet resolution on health and medical strategies. This has clarified the promotion of innovation in regenerative medicine and cell therapies under a public–private framework established by the Japanese government. In particular, the establishment of AMED is a centerpiece of this policy. Modelled on the US National Institutes of Health, AMED is an attempt to centralize bio and healthcare subsidies/grants that were previously divided among several ministries/government agencies and to emphasise patient-centred innovation.
The second related policy is to push forward initiatives for banking pluripotent stem cells (iPSCs). With regard to iPSCs, the Center of iPS Cell Research (CiRA) at Kyoto University established the Facility for iPS Cell Therapy (FiT) [7] and announced a policy of using blood banks and umbilical cord blood banks for iPSC stocks. Although this initiative involves only a single university, CiRA is expected to lead innovation in regenerative medicine and cell therapies. It has received ¥110 billion in aid from government funding agencies, has been designated as an iPSC supply base for Japan, and is also participating in an initiative for international standardization.
The third related policy is to promote regional innovation through the establishment of Comprehensive Special Zones (CSZ) [8]. The CSZs were established in 2013 and include the formation of industrial and functional concentration points intended to serve as core drivers of Japan’s economic growth. Among these, the Life Innovation in Keihin Coastal Areas CSZ for International Competitiveness is a remarkable model of innovation in regenerative medicine and cell therapies [8].
Finally, it should be mentioned that the establishment of this legal framework has promoted easing of regulations with regard to gene therapies as well. The definition of regenerative medicine covers a wide range of disciplines, and in the future we can expect integrated development of cell and gene therapies, such as cancer immunotherapy. The recent legal and regulatory changes treat these new therapies as subjects. Table 1 illustrates this idea as a matrix.
| Regenerative medicine | Cell and gene therapies other than the definition of regenerative medicine | |
|---|---|---|
| RMS Act | Regenerative medicine therapies including using human stem cells | Immunotherapies using dendritic cells |
| PMD Act | Regenerative medicine products including using human stem cells | Innovative pharmaceutical products and medical devices, including cell-based ones |
Company case details
Under the PMD Act, regenerative medicine products can be approved at an early stage, with conditions and time limits, if their effectiveness has been assumed and their safety has been confirmed. Table 2 shows a list of products and clinical programs in the fields of regenerative medicine and cell and gene therapies that were announced in Japan in May 2015. Thus far few clinical programs are being developed with a view to applying under the early approval system. In the last year applications were submitted for an allogeneic mesenchymal stem cell product JR-031 (JCR Pharmaceuticals Co., Ltd) [9] and autologous skeletal myoblast sheets (Terumo Corporation) [10]; these were the first cell therapy drug and regenerative medicine therapy to be approved under the PMD Act. However, if the company considers that the National Health Insurance Reimbursement Price applied after approval will not be profitable, the company may choose not to market the product under the National Health Insurance System, even if it has been approved. The manner in which the National Health Insurance Reimbursement Price is determined is worthy of further consideration.
Table 2 does not include items of investigator-initiated clinical research. However, biotechnology companies are advancing developments aimed at obtaining approvals, such as iPSC-derived retina pigment epithelium cells HLCR011 (Healios K.K.) [11] for the treatment of age-related macular degeneration, and autologous oral mucosal epithelial sheets REG401 (Regience K.K.) for the treatment of refractory keratoconjunctive disease [12].
| Therapy | Product Name/ Development Code | Indication | Stage | Filing | Approval | NHI Reimburse-ment | Development Company |
|---|---|---|---|---|---|---|---|
| Autologous cultured epidermal cell sheets | JACE | Serious burns | Marketed | Oct. 2004 | Jul. 2007 | Jan. 2009 | Japan Tissue Engineering |
| Bullous epidermolysis | NDA | ||||||
| Giant pigmented nevus | P2/3 | ||||||
| Autologous cultured cartilage cells | JACC | Relief of symptoms of traumatic cartilage defects and osteochondritis dissecans (exclude osteoarthritis) for knee joints | Marketed | Aug. 2009 | Jul. 2012 | Apr. 2013 | Japan Tissue Engineering |
| Allogeneic human mesenchymal stem cells | JR-031 | Suppression of graft-versus-host disease | NDA | Sep. 2014 | JCR Pharma (Licensor: Osiris Therapeutics) | ||
| Autologous skeletal myoblast sheets | Severe heart failure | NDA | Oct. 2014 | Terumo | |||
| MAGE-A4 antigen-specific T cell receptor gene therapy | Oesophageal cancer, etc. | P1 | Takara Bio | ||||
| NY-ESO-1 antigen-specific T cell receptor gene therapy | Synovial sarcoma, etc. | P1 | Takara Bio | ||||
| Autologous cultured corneal epithelial cell sheets | Corneal epithelial stem cell deficiency | IND | Japan Tissue Engineering/ CellSeed | ||||
| CD19 antigen specific Chimeric Antigen Receptor gene therapy | B cell non-Hodgkin Lymphoma, etc. | Preparation for P1 | Takara Bio | ||||
| Dendritic cell vaccine therapy | Vaccell | Cancer | Preparation for P1 | Tella Pharma |
The trends at non-Japanese corporations with respect to the PMD Act have yet to emerge. However, Mesoblast Ltd, which has acquired the cell therapy business of Osiris Therapeutics Inc., including JR-031, indicated in the CEO presentation at its general shareholders meeting in November 2014 that it is taking steps to achieve early market launch in Japan of its clinical development-stage cell therapies, including recruiting personnel in Japan and continuing discussions with the Pharmaceuticals and Medical Devices Agency (PMDA) [13]. Given the above situation, it seems likely that many products, conditionally time-limited-approved items and clinical programs will be reported in the near future.
With the enforcement of the RMS Act, it has now become possible to outsource cell culturing processes. As a result, Takara Bio Inc. [14] and MEDINET Co., Ltd [15], which have expertise in cell culturing, have announced that they have obtained a license as specific processed cells manufacturers to build a new cell processing facility in May 2015 (Table 3) [16]. Furthermore, the optical equipment manufacturer Nikon Corporation announced that it has made a strategic business alliance agreement with Lonza of Switzerland, a major producer of cells for regenerative medicine, relating to outsourced cell production in Japan [17]. Nikon will enter the business of outsourced production of cells for regenerative medicine through a newly established wholly owned subsidiary. Thus, with the enactment of the RMS Act, cell processing outsourcing appears to have emerged as a new business opportunity.
| Company | Outline | Timing of operation | Facility Scale (gross floor area) | Location | Notes |
|---|---|---|---|---|---|
| Takara Bio | Establishment of the Center for Gene and Cell Processing | Oct. 2014 | Approx. 6,500 m2 | Kusatsu, Shiga | Permission for processing of specific cells (May 2015) |
| MEDINET | Establishment of the Center for Cell Processing | 2015 | 2,990.5 m2 | Shinagawa, Tokyo | Permission for processing of specific cells (May 2015) |
| Nikon | Establishment of a 100% subsidiary with a cell processing centre | Second Half of FY2015 | Tokyo/Yokohama area under consideration | A strategic collaboration agreement (Facility Support and License Agreement) with Lonza (Switzerland) was signed (May 2015) |
Furthermore, categorizing the main initiatives among Japanese pharmaceutical companies reveals three trends:
- To establish a regenerative medicine research unit either in-house or at a subsidiary;
- To promote joint research with academia;
- To in-license a clinical development program for regenerative medicine from biotechnology companies.
We will discuss some examples of these three trends in more detail.
1. To establish a regenerative medicine research unit either in-house or at a subsidiary
In April 2015, to reinforce research to evaluate the regenerative medicine programs for clinical studies and create a structure capable of more efficiently and effectively managing external collaboration with regenerative medicine-related authorities, as well as internal collaboration, Astellas Pharma Inc. reorganized the Regenerative Medicine Unit established in April 2014 as the Regenerative Medicine Labs [18]; in May 2015 Rohto Pharmaceutical Co., Ltd announced the establishment of a new subsidiary, INTERSTEM, for the management of patents relating to regenerative medicines as well as the management and out-licensing the outcomes from collaborative research [19].
2. To promote joint research with academia
Takeda released an announcement of its 10-year collaboration on iPSC research with CiRA of Kyoto University in April 2015 [20]. Rohto [21] and Astellas [22] established joint research chairs at The Center for Stem Cells and Regenerative Medicine at The Institute of Medical Science of The University of Tokyo and at the Graduate School of Medicine/Faculty of Medicine, Osaka University, respectively. Through academia–industry collaboration, Takeda intends to focus on the development of clinical applications of iPSCs in areas such as heart failure, diabetes mellitus, neurological disorders and cancer immunotherapy. The University of Tokyo and Rohto plan to conduct fundamental research to develop efficient methods for isolation from organs, induction of differentiation in a variety of cells and manufacturing of high-quality mesenchymal stem cells. Osaka University and Astellas plan to develop new technology platforms required to advance practical applications of cell therapies.
3. To in-license a clinical development program for regenerative medicine from biotechnology companies
SanBio Co., Ltd licensed SB623, a cell therapy for stroke, to Teijin Ltd for the Japanese market and Sumitomo Dainippon Pharma Co., Ltd. for North America, in February 2010 [23] and in September 2014 [24], respectively. The latter agreement was reached over 4 years under the option agreement signed in September 2010. More recently, in March 2015, Chugai Pharmaceutical Co., Ltd announced a license agreement and collaboration to develop MultiStem® Cell Therapy for ischemic stroke in Japan with Athersys, Inc., a biotechnology company based in the USA [25].
Some Japanese pharmaceutical companies are trying to incorporate the technology and knowledge required for the development of regenerative medicines and cell therapies into in-house research by accessing innovative scientific outcomes from academia. However, looking ahead, given the potential for rapid approval under the PMD Act, the in-licensing transactions in the Japanese market are expected to increase, similar to the deal between Chugai and Athersys.
In promoting regenerative medicine, cell therapy and gene therapy as businesses, it is necessary to revise regulations to reflect further technological improvements and the status of clinical work. This makes it essential for industry, academia and regulatory authorities to foster a close relationship.
Social impact and implications
The establishment of iPSCs in particular could be said to have instigated a boom in regenerative medicine and cell therapies in Japan. For example, in an internet survey to non-specialists [26], 87% of respondents claimed to be aware of regenerative medicine and 73% to know about iPSCs. Moreover, 90.3% of those respondents stated that regenerative medicine and iPSC research are necessary. Although this survey was conducted by a newspaper publisher, thus the potential bias of the respondent group needs to be considered, it is clear that as the population ages and the birth rate declines in Japan, the promotion of regenerative medicine and cell therapies is certain to be favoured.
However, while there is a high level of interest in regenerative medicine in Japan, as we have seen, it has not necessarily been implemented appropriately. As mentioned above, laws have been established to promote regenerative medicine and cell therapies; however, in 2010, prior to the enforcement of the law, a fatal medical accident occurred at a clinic in Kyoto following a surgical transplant of adipose-derived mesenchymal stem cells. Furthermore, a patient who received a mesenchymal stem cell transplant at another clinic in 2012 complained of lingering numbness as an after-effect and filed a lawsuit. The patient won the first hearing of the lawsuit in 2015, with the court recognizing that the clinic had failed to provide a sufficient explanation of the procedure. Despite this situation, Japanese advertisements for “stem-cell therapies” can be found on the internet, for beauty-enhancing procedures such as a breast implants and wrinkle removal. The Medical Care Act, which sets necessary rules concerning the establishment and management of medical institutions in Japan [27], states in Article 6-5 that “No party shall advertise matters other than the following with regard to a medical practice, dental practice, hospital, or clinic, whether in writing or by any other means”.
In light of the above situation, Medical Institution Website Guidelines have been formulated [28] and advertising regulations for private practices have finally been implemented. Looking ahead, to continue the promotion and spread of regenerative medicine research, it is necessary to focus on whether assessments by the Certified Special Committee for Regenerative Medicine have a scientific basis and include appropriate criteria from the perspective of patient protection [29]. Furthermore, it will be necessary to ensure that the registration system and advertising regulations for regenerative medicine activities prove effective.
As discussed above, regenerative medicine activities are now shifting to the clinical research phase, and rules are gradually being established regarding the question of how to protect patients, both physically and in terms of their rights, based on previous medical discussion on advanced medical research. However, questions remain regarding the capabilities that can be unleashed by the potential of iPSCs and ESCs, such as the propriety nature of research into the creation of reproductive cells and the creation of bodies through the combination of human cells and animal embryos to create whole organs, which imply the creation of human–animal chimeras, and other such questions relating to how we consider the nature of life itself. These issues must be addressed in order to deepen future research and ensure a diverse scope of applications.
These issues require extensive sharing of knowledge and discussion between experts and non-experts, rather than a hasty search for a solution. An awareness survey conducted for Japanese citizens in 2011 [30] found that there was a significant divide in the opinions of experts and non-experts. For example, regarding plans to create chimeric organisms from animal and human cells, among members of the Japanese Society for Regenerative Medicine, 71.5% of respondents approved of chimera creation (15.8% said they did not approve), whereas among non-experts, the approval rate was only 25.1%, with 45% saying that they did not approve.
The Ministry of Education, Culture, Sports, Science and Technology (MEXT) recognized the importance of conducting research into the ethical, legal and social issues surrounding regenerative medicine, allocating a portion of the funds for the Regenerative Medicine Realization Network project, one of the largest current expenditures related to regenerative medicine, to form a research group that will undertake survey research to anticipate ethical issues. At the same time, MEXT is preparing a framework for providing consultation based on research and medical ethics to medical researchers involved in clinical research. Even after the Regenerative Medicine Realization Network project is taken over by the AMED, this framework will be maintained.
Summary
The time is right for innovation in the fields of regenerative medicine and cell therapy in Japan, with a renovated regulatory framework that consists of the RMS Act for Regenerative Medicine Therapies and the PMD Acts for Regenerative Medicine Products.
Given the number of active clinical research initiatives in Japan, new regenerative medicine products are likely to require a few years before they receive tentative, expedited approval under the PMD act. Cell processing outsourcing appears to have been taken up as a new business opportunity with the enactment of the RMS Act.
We must focus on whether assessments have a scientific basis and meet appropriate criteria from the perspective of patient protection and ensure that the registration system and advertising regulations for regenerative medicine activities prove effective.
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.
Financial disclosure & acknowledgements
This work was partially supported by a grant from the New Energy and Industrial Technology Development Organization (FY2014; SS and MS) succeeded by Japan Agency for Medical Research and Development (FY2015; SS and MS). MS is currently an employee of CMIC HOLDINGS Co., Ltd. The authors have no other relevant affiliation with a financial interest in or financial conflict with the subject matter mentioned in this manuscript apart from those disclosed.
Affiliations
Shintaro Sengoku1,2,*
1 Graduate School of Innovation Management, Tokyo Institute of Technology
2 The Institute for Frontier Sciences, Kyoto University
* Correspondence addressed to: W9-114, 2-12-1 Ookayama, Meguro-ku, Tokyo 182-8552, Japan. sengoku@mot.titech.ac.jp
Mitsuya Sakurai3
3The Institute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University
Yoshimi Yashiro4
4 Uehiro Research Division for iPS Cell Ethics, Center for iPS Cell Research and Application (CiRA), Kyoto University
References
1. Watatani K, Xie Z, Nakatsuji N & Sengoku S. Global competencies from regional stem cell research: Bibliometrics for investigating and forecasting research trends. Regen Med. 2013; 8(5):659–668. CrossRef
2. Nakatsuji N. Irrational Japanese regulations hinder human embryonic stem cell research. Nat. Rep. Stem Cells 2007;
CrossRef
3. Konomi K, Tobita M, Kimura K & Sato D. New Japanese initiatives on stem cell therapies. Cell Stem Cell 2015; 16(4): 350-2. CrossRef
4. Hara A, Sato D & Sahara Y. A new governmental regulatory system for stem cell-based therapies in Japan. 2014. CrossRef
5. Azuma K. Regulatory landscape of regenerative medicine in Japan. Curr. Stem Cell Rep. 2015. CrossRef
6. Japan Agency for Medical Research and Development. www.amed.go.jp/en/
7. Stock of iPS Cells for Regenerative Medicine [cited 2015 June 20]. Center for iPS Cell Research and Application, Kyoto University. www.cira.kyoto-u.ac.jp/e/research/stock.html
8. Comprehensive Special Zones [cited 2015 June 20]. Prime Minister of Japan and His Cabinet. www.kantei.go.jp/jp/singi/tiiki/sogotoc/siryou/gaiyoueng.pdf
9. GlobeNewswire.com. Los Angeles: GlobeNewswire, Inc. [cited 2014 Sept. 30]. Website
10. terumo.com; Tokyo: Terumo Corporation [cited 2014 Oct. 31]. www.terumo.com/about/pressrelease/2014/20141031.pdf
11. healios.co.jp; Tokyo: Healios KK. www.healios.co.jp/en/development/pipeline/
12. regience.jp; Tokyo: Regience KK. http://regience.jp/english/rd-pipeline/product-development/#link01
13. mesoblast.com; Melbourne: Mesoblast Limited [cited 2014 Nov. 18]. http://ir.mesoblast.com/Investors/?page=presentations-webcasts
14. takara-bio.com; Otsu: Takara Bio Inc. www.takara-bio.com/overview/outline.htm
15. medinet-inc.co.jp; Yokohama: MEDINET Co., Ltd. www.medinet-inc.co.jp/english/company/profile.html
16. mhlw.go.jp;. Tokyo: Ministry of Health, Labour and Welfare. PDF
17. nikon.com; Tokyo: Nikon Corporation [cited 2015 May 7]. www.nikon.com/news/2015/0507_01.htm
18. astellas.com; Tokyo: Astellas Pharma Inc. [cited 2015 Feb. 5]. www.astellas.com/en/corporate/news/pdf/150205_2_Eg.pdf
19. rohto.co.jp; Osaka: ROHTO Pharmaceutical Co., Ltd. [cited 2015 May 14]. Available in Japanese PDF
20. takeda.com; Osaka: Takeda Pharmaceutical Co., Ltd. [cited 2015 April 17]. www.takeda.com/news/2015/20150417_6964.html
21. rohto.co.jp; Osaka: ROHTO Pharmaceutical Co., Ltd. [cited 2014 Oct. 9]. Available in Japanese from: www.rohto.co.jp/news/release/2014/1009_01/
22. astellas.com; Tokyo: Astellas Pharma Inc. [cited 2015 Feb. 2]. www.astellas.com/en/corporate/news/pdf/150202_2_eg.pdf
23. teijin.com; Osaka/Tokyo: Teijin Ltd. [cited 2010 Feb. 3]. www.teijin.com/news/2010/ebd100203_03.html
24. ds-pharma.com; Osaka: Sumitomo Dainippon Pharma Co., Ltd. [cited 2014 Sept. 26]. www.ds-pharma.com/news/2014/20140926.html
25. chugai-pharm.co.jp; Tokyo: Chugai Pharmaceutical Co., Ltd. [cited 2015 March 2]. www.chugai-pharm.co.jp/english/news/detail/20150302083000.html
26. Shineha R, Kawakami M, Kawakami K, Nagata M, Tada T & Kato K. Familiarity and prudence of the Japanese public with research into induced pluripotent stem cells, and their desire for its proper regulation. Stem Cell Rev. 2010; 6(1), 1-7. CrossRef
27. Japanese Law Translation. Website
28. Ministry of Health, Labor and Welfare. Available in Japanese PDF
29. Ministry of Health, Labor and Welfare. Available in Japanese PDF
30. Muto K, Available in Japanese from Website
