Enabling instrument connectivity through digital automation
Live30 webinars are thirty-minute presentations designed to update you on the latest innovations, applications, and data in a fast yet interactive format.
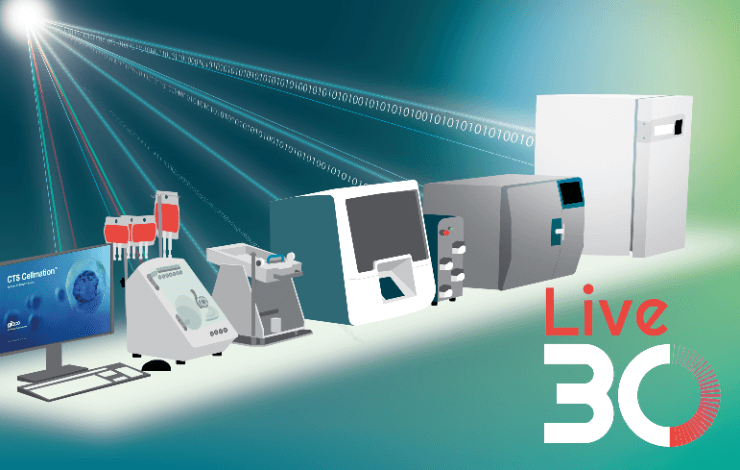
Automation of the manufacturing process for cell therapies could significantly increase manufacturing success rates, while reducing manual touchpoints and labor. Innovative instrumentation to address the various unit operations across the manufacturing process have been developed at Thermo Fisher Scientific, and are specifically designed with compatibility in mind. This facilitates digital integration and automation. Gibco™ CTS™ Cellmation™ Software for DeltaV™ System is an off-the-shelf digital solution, which allows users to connect their Thermo Fisher Scientific cell therapy instruments within a common DeltaV network to control workflows across multiple instruments in a 21 CFR Part 11-compliant environment.
In this webinar, we will introduce our closed, modular, end-to-end manufacturing process, as well as demonstrate how our instruments can be integrated,automated, and incorporated into existing workflows. You will gain insights into current challenges in the field that are being encountered, and how automation can help overcome these to improve the current state-of-the-art workflows.
- Introduction to CTS Cellmation software
- Importance of digital connectivity solutions to help ensure process and data integrity and integration of instruments into your DCS system
- Discuss existing challenges and the importance of automation and standardization within cell therapy manufacturing
You might also like
Next-gen cell therapy manufacturing: leveraging flexibility and automation for success
Enhancing manufacturing efficiency and quality through optimized lysis in plasmid DNA extraction

Faster, smaller, smarter: intelligent manufacturing of mRNA through the power of process analytical technologies
Transforming cell therapy manufacturing: smart automation & genetic modification for scalable success



