Delivering an end-to-end industrialization roadmap for autologous cell therapy
Today’s autologous cell therapy footprint has been expanding over recent years and the recent commercialization of certain CAR T therapies has further ignited the autologous growth across the globe. With hundreds of products in the pre-clinical and clinical phases, the industry has improved in both a quantitative and qualitative way, but has also been exposed to numerous pain points that have created critical bottlenecks affecting the ability for a smooth transition from clinic to market. From overall product cost of goods (COGs) to supply chain constraints, there is a critical need to industrialize the end-to-end process to allow for these life saving therapies to be commercialized and available to the global markets.
During this webinar, our experts reveal an end-to-end industrialization roadmap for drug developers of autologous cell therapies. This roadmap includes the key technologies, such as the Cocoon platform, necessary to achieve reliable COGs and scalability of these personalized medicines, as well as the partnerships required to build a supply chain network that will deliver ‘just-in-time’ manufacturing to patients.
- The specificities and challenges associated with autologous cell therapy manufacturing
- Leveraging automation for scalability of autologous cell therapy manufacturing with the Cocoon platform
- How to build a robust vein-to-vein network to achieve ‘just-in-time’ delivery of autologous cell therapies
You might also like
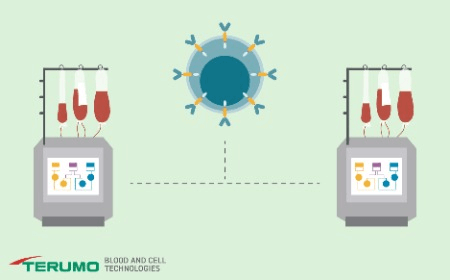
End-to-end automated manufacturing of low-seed CAR-T cells
Preserving the future of medicine: standardizing leukapheresis materials with cryopreservation for scalable cell therapies
Learn how to design a simplified, cost-effective LVV manufacturing workflow
When does outsourcing autologous collection center qualification and the cell therapy supply chain make sense?