Magnetic selection for consistent cellular starting material in autologous cell therapy manufacture
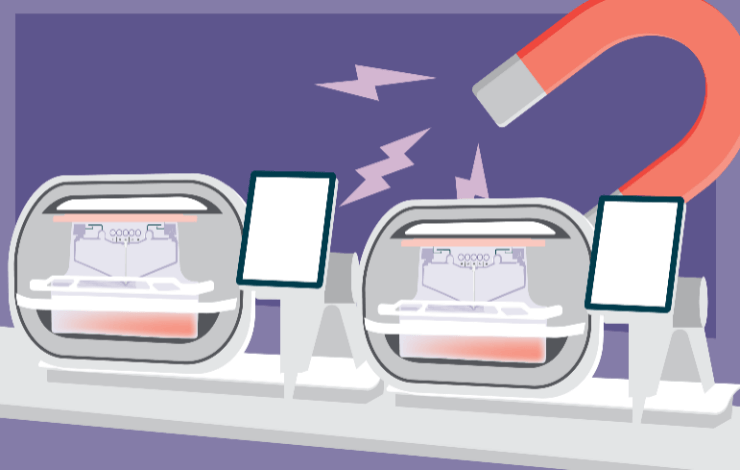
An efficient cell selection method is crucial to delivering a consistent autologous therapy product when the starting material received from severely ill patients is highly variable. While a variety of technologies are being adopted in the industry, there are very few GMP options, and many lack the flexibility for multiple passes, and flexibility in reagent selection. In addition, the currently available technologies are often applied in combination with manufacturing platforms that are manual, semi-automated and lacking commercial viability.
During this webinar, our experts will share insights on obtaining highly purified cells using magnetically active cell selection in a flexible, closed manufacturing system. In addition, we will learn about transitioning manual cell therapy production to automated processes that are scalable to meet clinical and commercial demands.
- Background, and the current status, of the Malaghan Institute’s CD19 cell therapy in a Phase I clinical trial
- Translation of Malaghan's open, manual manufacturing process into a fully automated workflow from sample loading, cell selection to harvest
- Magnetic selection in the Cocoon® Platform and results
You have registered for this webinar
You might also like

Strategic human raw material selection for cell therapy manufacturing
Advancing cell therapy: Optimizing the cell culture environment for high-quality manufacture of suspension and adherent cells

Manufacturing Quality Control for Plasmid DNA Critical Starting Materials
Preserving the future of medicine: standardizing leukapheresis materials with cryopreservation for scalable cell therapies



