Webinar topics
- Review the cell therapy cycle and learn how modular solutions enable successful cell therapy approaches
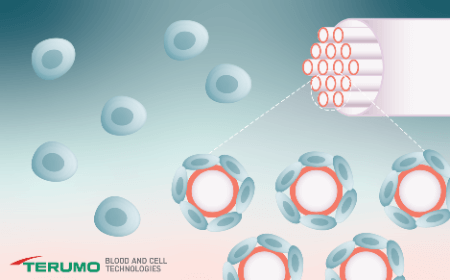
- Understand how suspension and adherent cells thrive by hollow-fiber perfusion technology
- Get to know Quantum Flex as the next generation of cell expansion facilitating GMP compliance
- Take a look into the lab to see Quantum Flex in action
- Review data of applications successfully cultured on the Quantum Flex platform
Why attend?
Over the past several years, cell therapy has seen explosive growth in the number and variety of applications and disease targets under exploration for commercialization, a trend that is unlikely to slow. The diversity of manufacturing strategies and technology to support this growth has also evolved, albeit more slowly. Cell therapy manufacturers now face a wider range of decisions when it comes to implementing automated solutions in their process. Committing to the wrong manufacturing technology early in commercialization can mean expensive, risky delays with late process transfers and comparability studies.
Key steps in the cell therapy manufacturing process include cell collection, genetic modification, cell expansion, formulation and fill and finish, all of which can benefit from modular automation whereby an equipment performs one task as part of a series and can integrate easily into an established or growing manufacturing process as a result of its simplicity and flexibility.
In this webinar we will introduce Terumo BCT’s new cell expansion platform: Quantum Flex. Building on the success and performance of hollow-fiber perfusion technology, Quantum Flex provides an optimized cell culture environment for adherent and suspension cell expansion, where cells thrive. Quantum Flex also features advanced software to support cGMP compliance, with user authentication, batch records and fleet management capabilities. This platform has the flexibility to process autologous and allogenic applications, as well as viral vector and exosome production, across multiple volumes of bioreactor, and showcases the scalability and connectivity to grow with your therapy, from process development to clinical manufacturing.

Hugo Fabre
Medical Science Liaison, Terumo BCT
Dr. Hugo Fabre joined Terumo BCT as Medical Science Liaison for Cell Collections and Cell Therapy Technology in February 2021. He is responsible for medical and scientific support, in-house or external associates training, stakeholder engagement and management as well as business intelligence related tasks.
Dr. Fabre has a PhD in Tissue Engineering and a Master’s degree in Cell Biology & Genetics. He gained knowledge as a researcher and entrepreneur from 10 years of translational research experience in mid-sized and start-up CROs as well as in academic and hospital settings with comprehensive intercultural and international experiences in global teams. He is recognized as a stem cells characterization specialist through the use of proprietary protocols for GMP-compliant and state of the art flow and image cytometry. He was also the CEO and co-founder of a Luxembourg-based cryptocurrency management fund that was successfully sold in 2019. Before joining Terumo BCT, Dr. Fabre was the Head of Business Development at Firalis, a mid-sized CRO and R&D company in the Basel area bringing lncRNA diagnostics solutions to market for a variety of neurologic and cardiac diseases.

Maura Barbisin
Field Application Scientist, Terumo BCT
Maura Barbisin joined Terumo BCT in 2018 as Field Application Scientist in EMEA to support customers in the Cell and Gene Therapy field and facilitate their process automation. In her role, she provides full technical training on Terumo BCT Cell Therapy device suite and continuous scientific support during device evaluations and post-sale implementations.
Previously, she worked as Sr. Staff Scientist R&D at Applied Biosystems-Life Technologies, now part of Thermo Fisher (Foster City, CA, USA) for 10 years with increasing responsibilities from individual contributor to project leader and then also people manager, developing products based on qPCR and sequencing technologies. Among other roles, Maura Barbisin worked as contract researcher at SISSA-International School for Advanced Studies (Trieste, Italy) with a research assignment in the Neuroscience Department-Prion Biology Laboratory to study the whole transcriptome of animal models affected by prion diseases. Furthermore, she was a Scientist at ISS-Istituto Superiore di Sanità (Rome, Italy) in the Virology department-HIV Laboratory to evaluate the efficacy of a protein-based HIV vaccine. She studied the role of cell-mediated immune response during vaccination in animal models.
Maura Barbisin holds an M. Sc. in Biological Sciences from the University of Trieste, Italy. She is an experienced molecular and cell biologist with extensive knowledge of both academia and biotech/medical device industry.
Maria Knaub
Field Application Scientist, Terumo BCT
Dr. Maria Knaub joined Terumo BCT in 2021 as a Field Application Scientist for Cell Therapy Technologies in EMEA to support customers in their process automation. In her role, she provides full technical training on Terumo BCT Cell Therapy device suite and continuous scientific support of customers during device evaluations and post-sale implementations. For the Quantum Flex launch, she applies her cross-functional knowledge and experience to support globally.
Dr. Maria Knaub studied biomedical chemistry in Mainz, Germany, and worked in parallel as a paramedic and first aid instructor. In Heidelberg, Germany, she completed her PhD in molecular biology, where she focused signalling pathways in liver cancer development and supervised interns and trainees. Afterwards, she worked as a project manager, where she led different international customer projects in GxP environment and mentored new employees into their roles.