From process development to manufacturing: how to successfully optimize and scale up the AAV enrichment step on novel CIMmultus QA HR line
Adeno-associated viruses (AAV) have emerged as leading vectors for gene therapy applications due to their low pathogenicity and ability to mediate long-term gene expression in a wide range of tissues. In each AAV downstream process, one of the key steps (and often a bottleneck) is enrichment of full capsids. Along with scalability and capsid separation challenges, one should account for sample heterogeneity and assess which analytics are adequate to distinguish between AAV sub-populations and deliver only the potent AAV product.
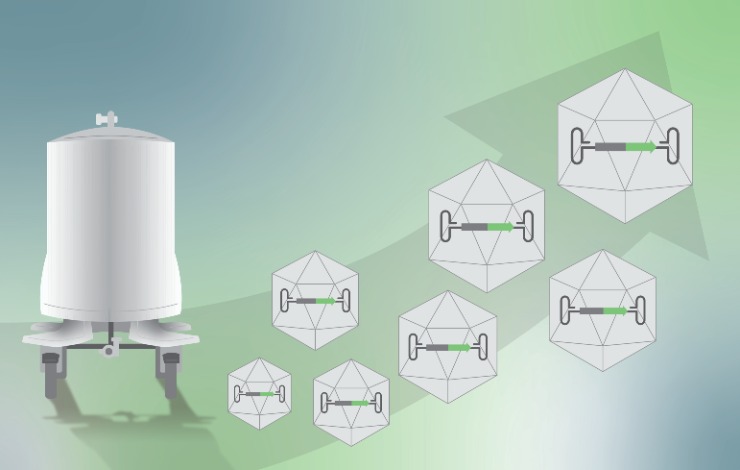
To address these needs, Sartorius BIA Separations developed new CIMmultus HR (High Reproducibility) line columns with better resolution and narrower acceptance criteria. These columns allow for batch-to-batch and scale-to-scale reproducibility within just 3% of the AAV8 empty capsid iso-conductivity elution. Such columns allow for step gradient elution using the same buffer strength at any scale. Two order of magnitude removal of empty capsids should be possible.
A complete enrichment process design on AAV8 case study will be presented., starting from initial screening of conditions using 96-well plate or small monolithic columns, followed by the determination of column capacity, considerations for formation of step gradient, and finally a successful scale-up.
Join us in this webinar to learn about:
- Chromatographic purification of AAV
- Development of the enrichment step
- Improving resolution and reproducibility with the CIMmultus HR line
- Successful scale up
You might also like
Meeting manufacturing demand: robust scale-up of an AAV suspension process to 1,000L
Meeting manufacturing demand: robust scale-up of an AAV suspension process to 1,000L

Mastering AAV production: best practices For small- to large-scale manufacturing success and reduced development time



