Development of a non-viral gene delivery platform for CAR-T manufacturing
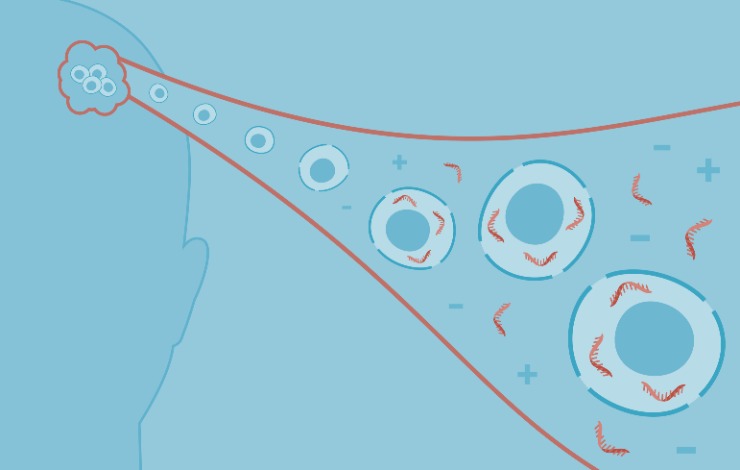
The use of CAR-T cells as potent therapies for refractory B-cell and plasma cell malignancies poses an immense financial burden. It requires lengthy manufacturing times, especially for GMP-grade vector production.
Non-viral gene delivery methods are therefore a desirable alternative, and the first non-viral cell therapy was recently FDA-approved. Homology-directed recombination (HDR) is a common strategy for gene delivery, but its low frequency yields few CAR-T cells during manufacturing. In contrast, non-homologous end joining (NHEJ) is the primary pathway of dsDNA break repair, and NHEJ-mediated homology-independent targeted insertion (HITI) has been previously shown to more efficiently knock-in targets as compared to HDR in several cell types and pre-clinical studies.
Here, we show HITI-mediated site-directed integration of a therapeutically relevant GD2-CAR transgene into the T cell receptor alpha constant (TRAC) locus using nanoplasmid DNA and CRISPR/Cas9 in primary human T cells. We will compare viral and non-viral platforms for developing a GD2 CAR-T manufacturing process while demonstrating process comparability and non-viral process feasibility. We will provide a deeper understanding of how to improve cell engineering efficiencies, cell viabilities, and scalability.
- Explore the cutting-edge techniques employed by leading institutions increasing CAR knock-ins in T cells mediated by by homology-directed repair (HDR) or homology-independent targeted insertion (HITI)
- Explore how selecting a clinically-validated manufacturing platform for crucial processes like cell electroporation can mitigate risks in cell therapy production
- Gain a deeper understanding of how to use a non-viral process for CAR-T manufacturing
You might also like
Enhancing CAR-T cell generation: optimizing non-viral engineering of resting T cells for improved cancer immunotherapy
Clinical-scale non-viral gene edited CAR-NK cells for cell therapy

Overcoming the limits of lentivirus: de-risk cell therapy development with non-viral genome engineering




